GROUP I | Invacare® Therapeutic Support Surfaces | ||
|
|
|
|
|
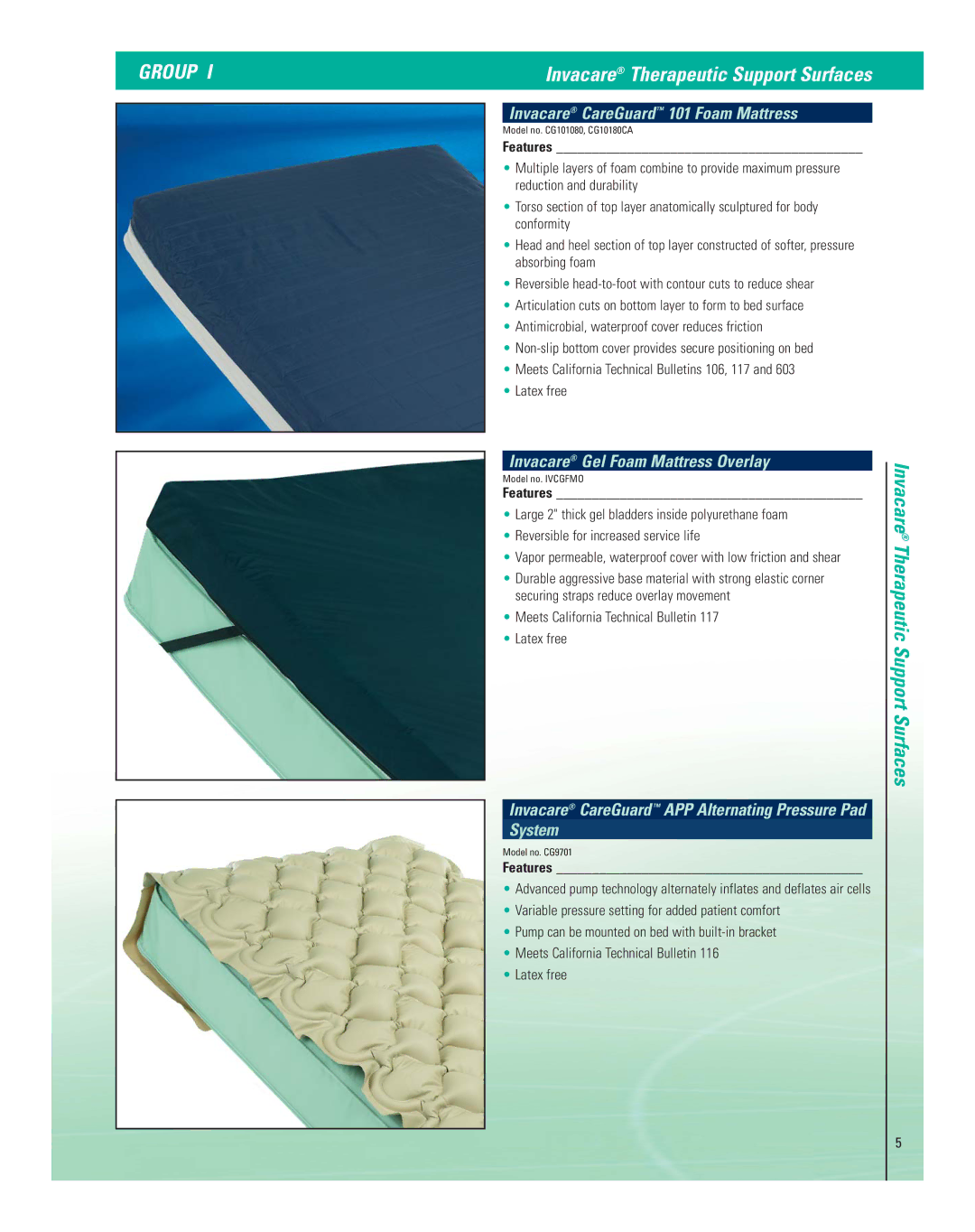
| Invacare® CareGuard™ 101 Foam Mattress |
|
|
| Model no. CG101080, CG10180CA | |
|
| Features ___________________________________________ | |
|
| • Multiple layers of foam combine to provide maximum pressure | |
|
| reduction and durability | |
|
| • Torso section of top layer anatomically sculptured for body | |
|
| conformity | |
|
| • Head and heel section of top layer constructed of softer, pressure | |
|
| absorbing foam | |
|
| • Reversible | |
|
| • Articulation cuts on bottom layer to form to bed surface | |
|
| • Antimicrobial, waterproof cover reduces friction | |
|
| • | |
|
| • Meets California Technical Bulletins 106, 117 and 603 | |
|
| • Latex free | |
|
|
|
|
Invacare® Gel Foam Mattress Overlay
Model no. IVCGFMO
Features ___________________________________________
•Large 2" thick gel bladders inside polyurethane foam
•Reversible for increased service life
•Vapor permeable, waterproof cover with low friction and shear
•Durable aggressive base material with strong elastic corner securing straps reduce overlay movement
•Meets California Technical Bulletin 117
•Latex free
Invacare® CareGuard™ APP Alternating Pressure Pad System
Model no. CG9701
Features ___________________________________________
•Advanced pump technology alternately inflates and deflates air cells
•Variable pressure setting for added patient comfort
•Pump can be mounted on bed with
•Meets California Technical Bulletin 116
•Latex free
Invacare® Therapeutic Support Surfaces
5