NICKEL METAL HYDRIDE BATTERIES - CONTINUED
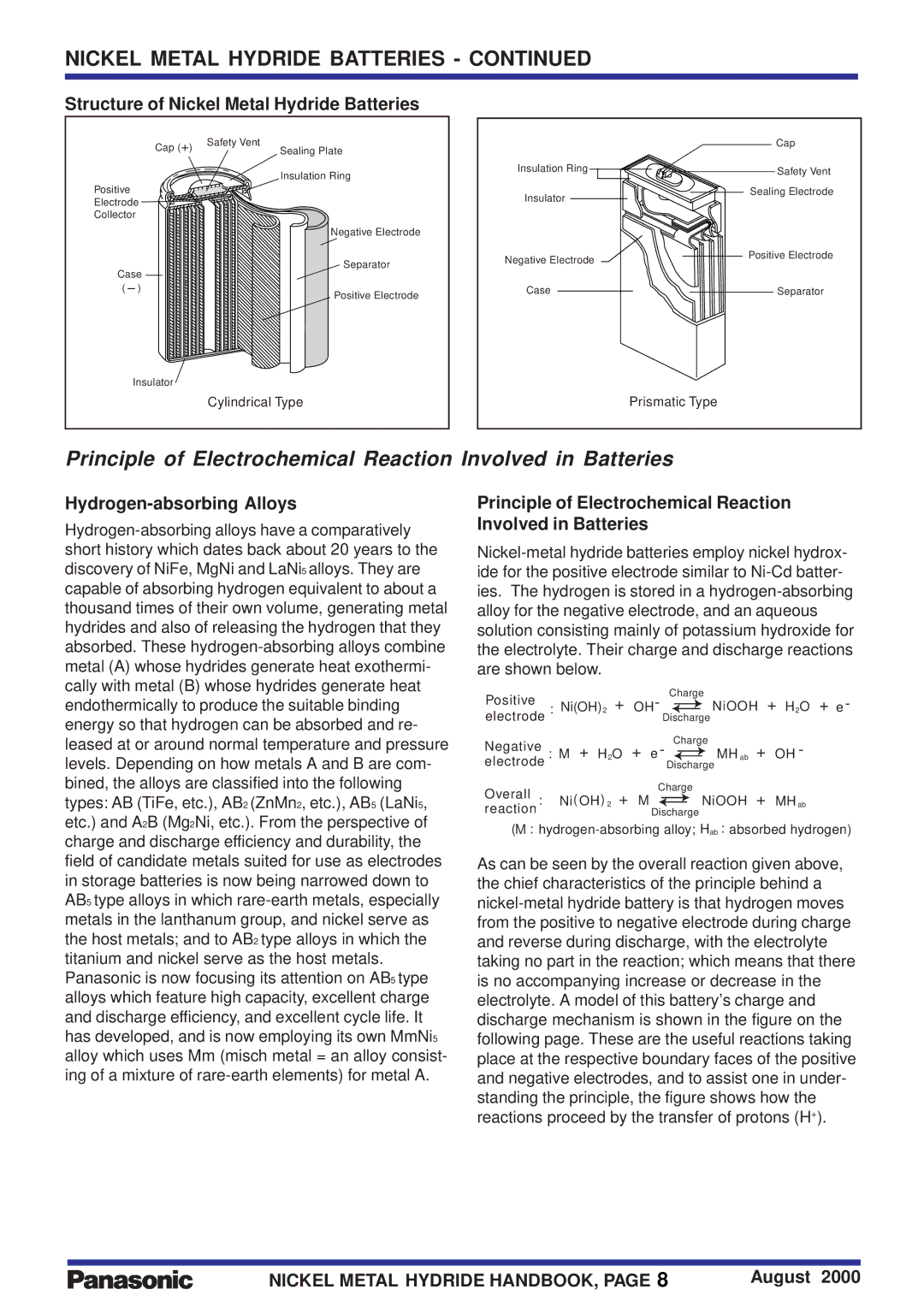
Structure of Nickel Metal Hydride Batteries
Cap (+) | Safety Vent |
Sealing Plate |
| Insulation Ring |
Positive | |
Electrode | |
Collector | |
Negative Electrode
Cap
Safety Vent Sealing Electrode
Separator
Positive Electrode
Positive Electrode
Separator
Insulator 
Cylindrical Type
Principle of Electrochemical Reaction Involved in Batteries
Hydrogen-absorbing Alloys
Hydrogen-absorbing alloys have a comparatively short history which dates back about 20 years to the discovery of NiFe, MgNi and LaNi5 alloys. They are capable of absorbing hydrogen equivalent to about a thousand times of their own volume, generating metal hydrides and also of releasing the hydrogen that they absorbed. These hydrogen-absorbing alloys combine metal (A) whose hydrides generate heat exothermi- cally with metal (B) whose hydrides generate heat endothermically to produce the suitable binding energy so that hydrogen can be absorbed and re- leased at or around normal temperature and pressure levels. Depending on how metals A and B are com- bined, the alloys are classified into the following types: AB (TiFe, etc.), AB2 (ZnMn2, etc.), AB5 (LaNi5, etc.) and A2B (Mg2Ni, etc.). From the perspective of charge and discharge efficiency and durability, the field of candidate metals suited for use as electrodes in storage batteries is now being narrowed down to AB5 type alloys in which rare-earth metals, especially metals in the lanthanum group, and nickel serve as the host metals; and to AB2 type alloys in which the titanium and nickel serve as the host metals. Panasonic is now focusing its attention on AB5 type alloys which feature high capacity, excellent charge and discharge efficiency, and excellent cycle life. It has developed, and is now employing its own MmNi5 alloy which uses Mm (misch metal = an alloy consist- ing of a mixture of rare-earth elements) for metal A.
Principle of Electrochemical Reaction Involved in Batteries
Nickel-metal hydride batteries employ nickel hydrox- ide for the positive electrode similar to Ni-Cd batter- ies. The hydrogen is stored in a hydrogen-absorbing alloy for the negative electrode, and an aqueous solution consisting mainly of potassium hydroxide for the electrolyte. Their charge and discharge reactions are shown below.
Positive | | | | | | Charge | | | |
| | | | | | | | | | | | | | | | |
: Ni(OH)2 | + | OH- | | | | | | | | NiOOH | + H2O + e - |
electrode | | | | | Discharge | | | |
Negative | : M + H2O | + | e - | Charge | | | MH ab | + | OH - |
| |
electrode | | | | | | |
| | | | | Discharge | | |
Overall : | Ni( OH) 2 | + | M | Charge | | + MH ab |
| | | | | | | | | NiOOH |
| | | | | | | | |
| | | | | | | | |
reaction | | | | Discharge | | | |
(M : hydrogen-absorbing alloy; Hab : absorbed hydrogen)
As can be seen by the overall reaction given above, the chief characteristics of the principle behind a nickel-metal hydride battery is that hydrogen moves from the positive to negative electrode during charge and reverse during discharge, with the electrolyte taking no part in the reaction; which means that there is no accompanying increase or decrease in the electrolyte. A model of this battery’s charge and discharge mechanism is shown in the figure on the following page. These are the useful reactions taking place at the respective boundary faces of the positive and negative electrodes, and to assist one in under- standing the principle, the figure shows how the reactions proceed by the transfer of protons (H+).
NICKEL METAL HYDRIDE HANDBOOK, PAGE 8 | August 2000 |

![]()