TRG-TRC003-EN 27
period four
Pressure–Enthalpy Chart
notes envelope, the refrigerant exists as a mixture of liquid and vapor. If the enthalpy
of the refrigerant lies to the right of the envelope, the vapor is superheated.
Similarly, if the enthalpy of the refrigerant lies to the left of the envelope, the
liquid is subcooled.
Lines of constant temperature cross the P–
h
chart as shown.
To further demonstrate the use of the P–
h
chart, let us look at the process of
heating and boiling water, at a constant pressure, on a P–
h
chart for water.
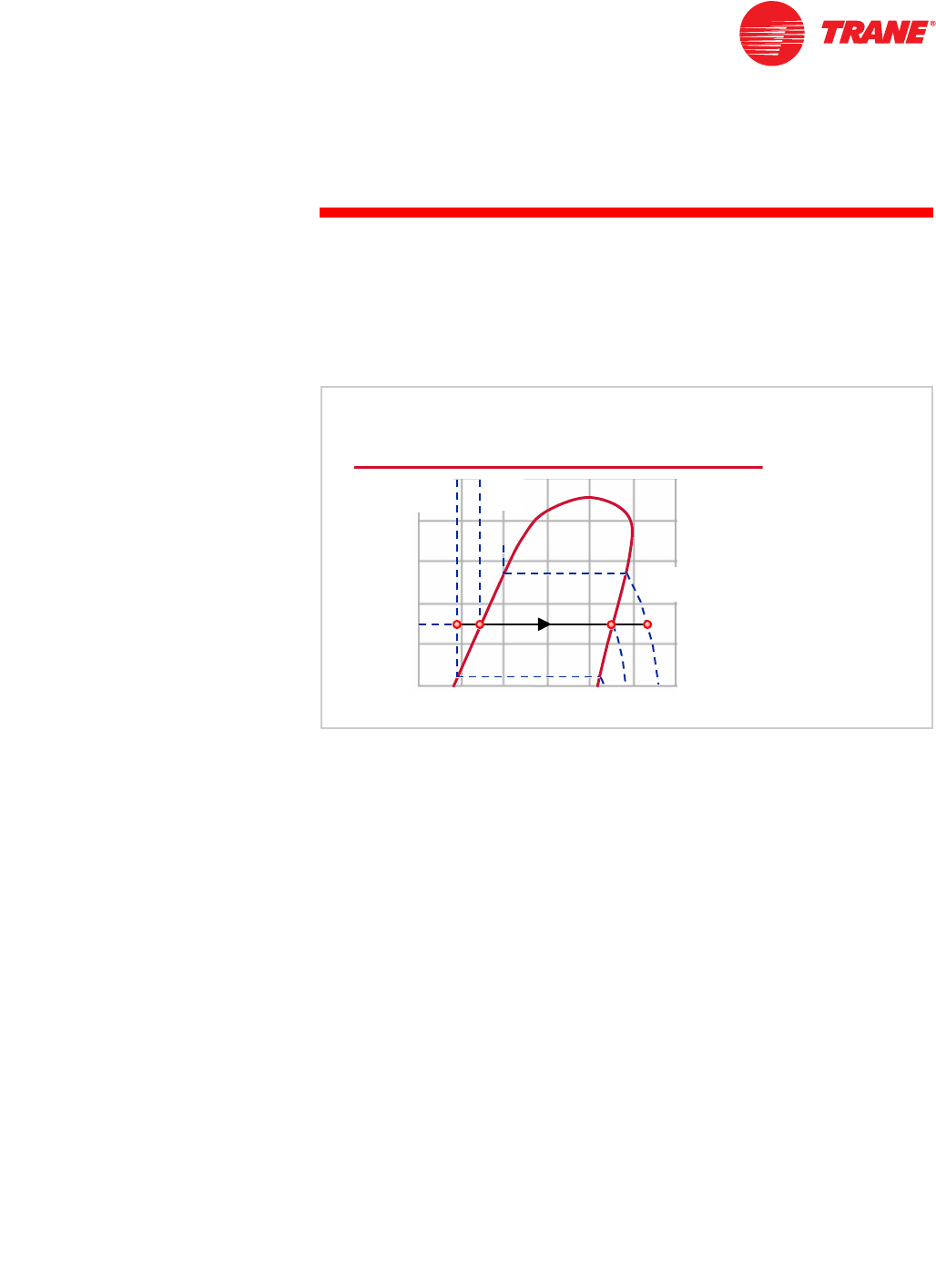
As discussed earlier, at atmospheric pressure (14.7 psia [0.10 MPa]) water boils
at 212°F [100°C]. At $, the water temperature is 180 °F [82.2°C]. As we add heat
to the water, the temperature and enthalpy of the water increas as they move
toward %. When the water reaches its saturated condition (%), at 212°F [100°C],
it starts to boil and transform into vapor. As more heat is added to the water, it
continues to boil while the temperature remains constant. A greater percentage
of the water is transforming into vapor as it moves toward &.
When the water reaches & on the saturation vapor line, it has completely
transformed into vapor. Now, as more heat is added to the vapor, its
temperature begins to increase again toward D, 240°F [115.6°C].
$
HQWKDOS\
HQWKDOS\
'
3¤K&KDUWIRU:DWHU
SVLD
SVLD
>
>03D
03D@
@
)
)
>&@
>&@
)
)
>&@
>&@
%&
)
)
>&@
>&@
SUHVVXUH
SUHVVXUH
:DWHU
:DWHU
Figure 42