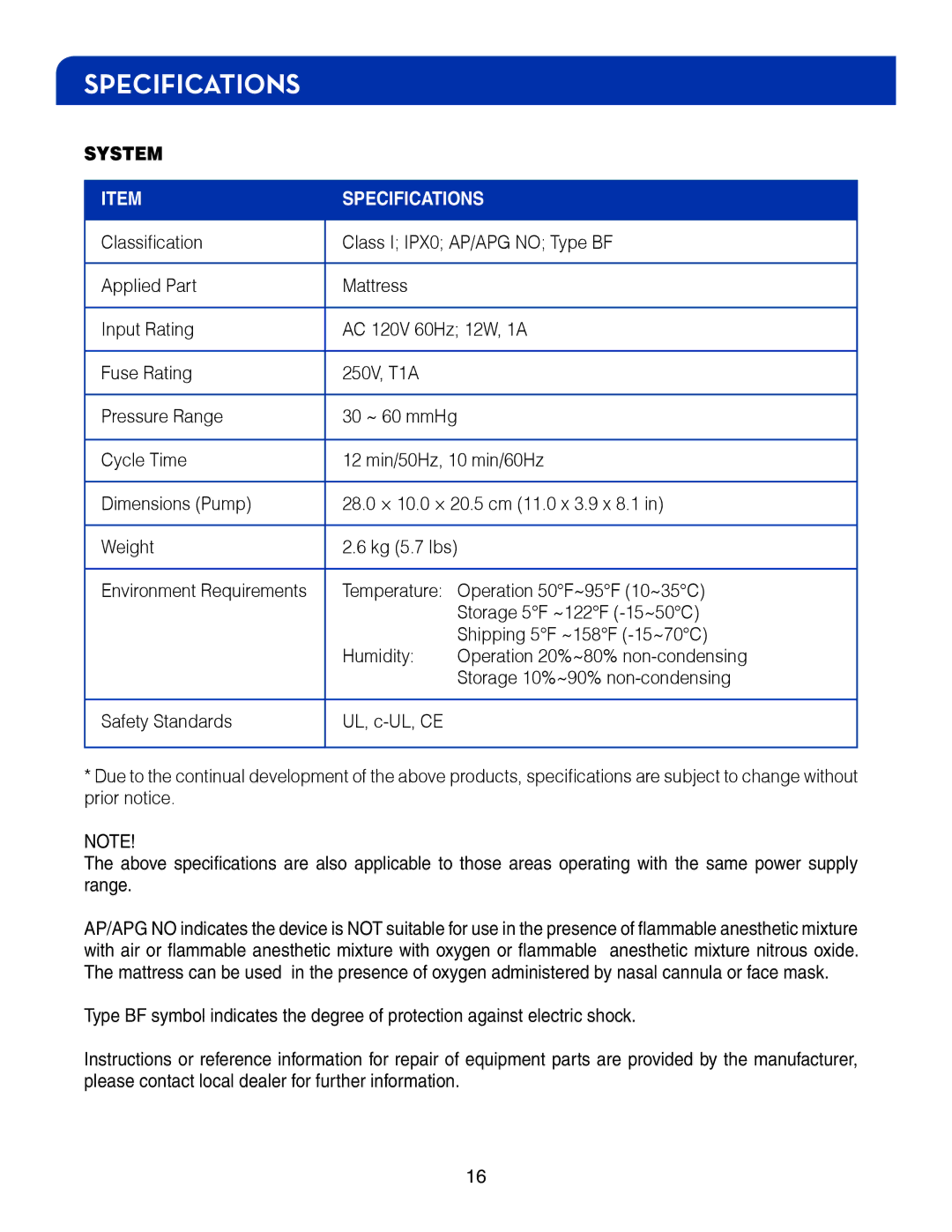
SPECIFICATIONS
SYSTEM
ITEM | SPECIFICATIONS | |
|
| |
Classification | Class I; IPX0; AP/APG NO; Type BF | |
|
|
|
Applied Part | Mattress |
|
|
| |
Input Rating | AC 120V 60Hz; 12W, 1A | |
|
|
|
Fuse Rating | 250V, T1A |
|
|
|
|
Pressure Range | 30 ~ 60 mmHg |
|
|
| |
Cycle Time | 12 min/50Hz, 10 min/60Hz | |
|
| |
Dimensions (Pump) | 28.0 × 10.0 × 20.5 cm (11.0 x 3.9 x 8.1 in) | |
|
|
|
Weight | 2.6 kg (5.7 lbs) |
|
|
|
|
Environment Requirements | Temperature: | Operation 50°F~95°F (10~35°C) |
|
| Storage 5°F ~122°F |
|
| Shipping 5°F ~158°F |
| Humidity: | Operation 20%~80% |
|
| Storage 10%~90% |
|
|
|
Safety Standards | UL, |
|
|
|
|
*Due to the continual development of the above products, specifications are subject to change without prior notice.
NOTE!
The above specifications are also applicable to those areas operating with the same power supply range.
AP/APG NO indicates the device is NOT suitable for use in the presence of flammable anesthetic mixture with air or flammable anesthetic mixture with oxygen or flammable anesthetic mixture nitrous oxide. The mattress can be used in the presence of oxygen administered by nasal cannula or face mask.
Type BF symbol indicates the degree of protection against electric shock.
Instructions or reference information for repair of equipment parts are provided by the manufacturer, please contact local dealer for further information.
16