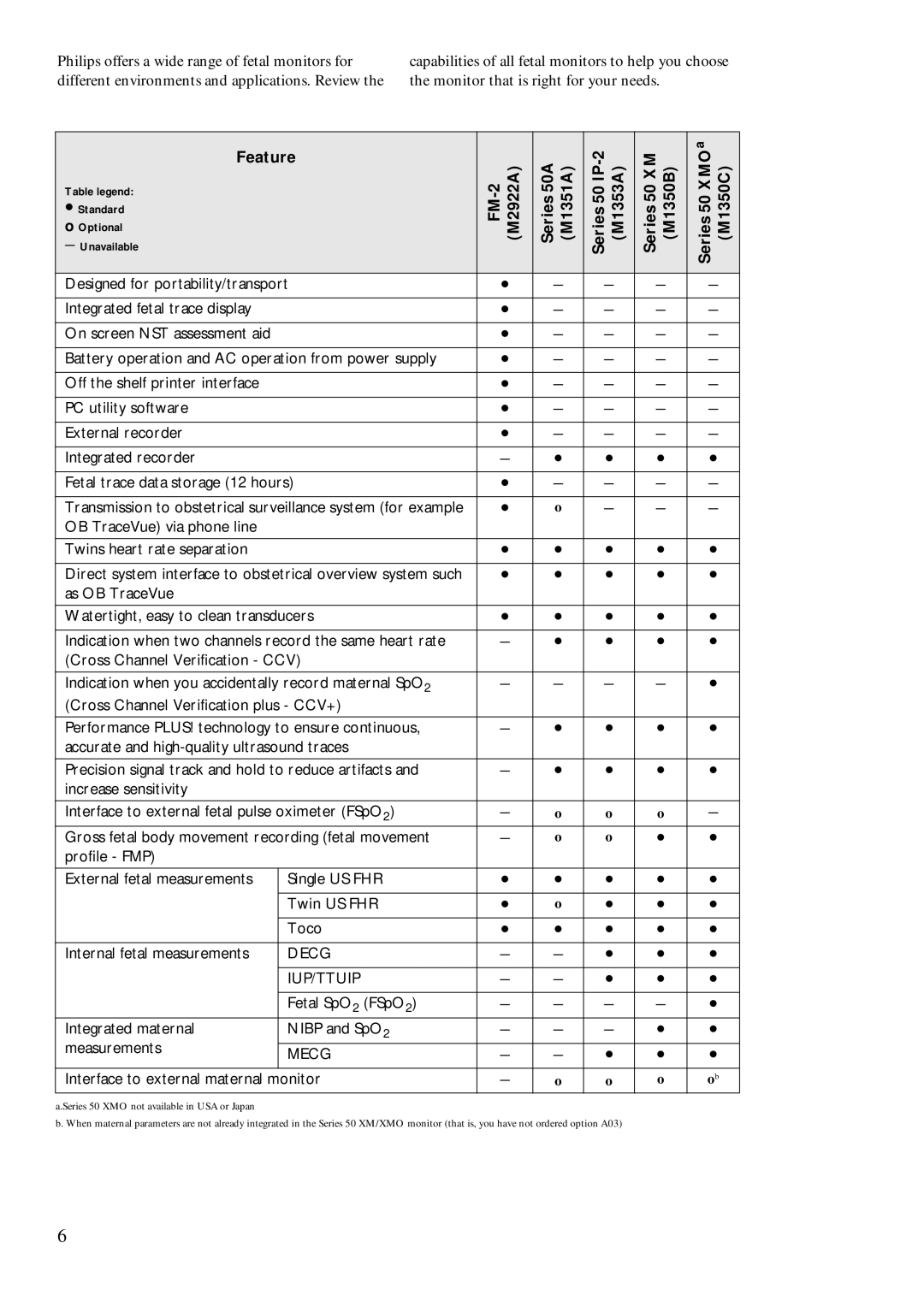
Philips offers a wide range of fetal monitors for different environments and applications. Review the
capabilities of all fetal monitors to help you choose the monitor that is right for your needs.
Feature |
|
|
|
|
| a | |
| (M2922A) | Series 50A (M1351A) | Series 50 | Series 50 XM (M1350B) | Series 50 XMO (M1350C) | ||
Table legend: |
|
| |||||
• Standard |
| ||||||
o Optional |
| ||||||
− Unavailable |
| ||||||
|
| ||||||
|
|
|
|
|
|
|
|
Designed for portability/transport |
| • | − | − | − | − | |
Integrated fetal trace display |
|
| • | − | − | − | − |
On screen NST assessment aid |
|
| • | − | − | − | − |
Battery operation and AC operation from power supply |
| • | − | − | − | − | |
Off the shelf printer interface |
|
| • | − | − | − | − |
PC utility software |
|
| • | − | − | − | − |
External recorder |
|
| • | − | − | − | − |
Integrated recorder |
|
| − | • | • | • | • |
Fetal trace data storage (12 hours) |
| • | − | − | − | − | |
Transmission to obstetrical surveillance system (for example |
| • | o | − | − | − | |
OB TraceVue) via phone line |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Twins heart rate separation |
|
| • | • | • | • | • |
Direct system interface to obstetrical overview system such |
| • | • | • | • | • | |
as OB TraceVue |
|
|
|
|
|
|
|
Watertight, easy to clean transducers |
| • | • | • | • | • | |
Indication when two channels record the same heart rate |
| − | • | • | • | • | |
(Cross Channel Verification - CCV) |
|
|
|
|
|
| |
Indication when you accidentally record maternal SpO2 |
| − | − | − | − | • | |
(Cross Channel Verification plus - CCV+) |
|
|
|
|
|
| |
|
|
|
|
|
|
| |
Performance PLUS! technology to ensure continuous, |
| − | • | • | • | • | |
accurate and |
|
|
|
|
|
| |
|
|
|
|
|
|
| |
Precision signal track and hold to reduce artifacts and |
| − | • | • | • | • | |
increase sensitivity |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Interface to external fetal pulse oximeter (FSpO2) |
| − | o | o | o | − | |
Gross fetal body movement recording (fetal movement |
| − | o | o | • | • | |
profile - FMP) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
External fetal measurements | Single US FHR |
| • | • | • | • | • |
| Twin US FHR |
| • | o | • | • | • |
| Toco |
| • | • | • | • | • |
Internal fetal measurements | DECG |
| − | − | • | • | • |
| IUP/TTUIP |
| − | − | • | • | • |
| Fetal SpO2 (FSpO2) |
| − | − | − | − | • |
Integrated maternal | NIBP and SpO2 |
| − | − | − | • | • |
measurements |
|
|
|
|
|
|
|
MECG |
| − | − | • | • | • | |
|
| ||||||
Interface to external maternal monitor |
| − | o | o | o | ob | |
a.Series 50 XMO not available in USA or Japan
b. When maternal parameters are not already integrated in the Series 50 XM/XMO monitor (that is, you have not ordered option A03)
6