solution. As more energy is drained from the battery, the ratio of sulfuric acid to water decreases and the created lead sulfate byproduct begins forming on the electrode plates. A low hydrometer reading means the chemical makeup that generates the free electrons is diminished so not as much energy is stored for use.
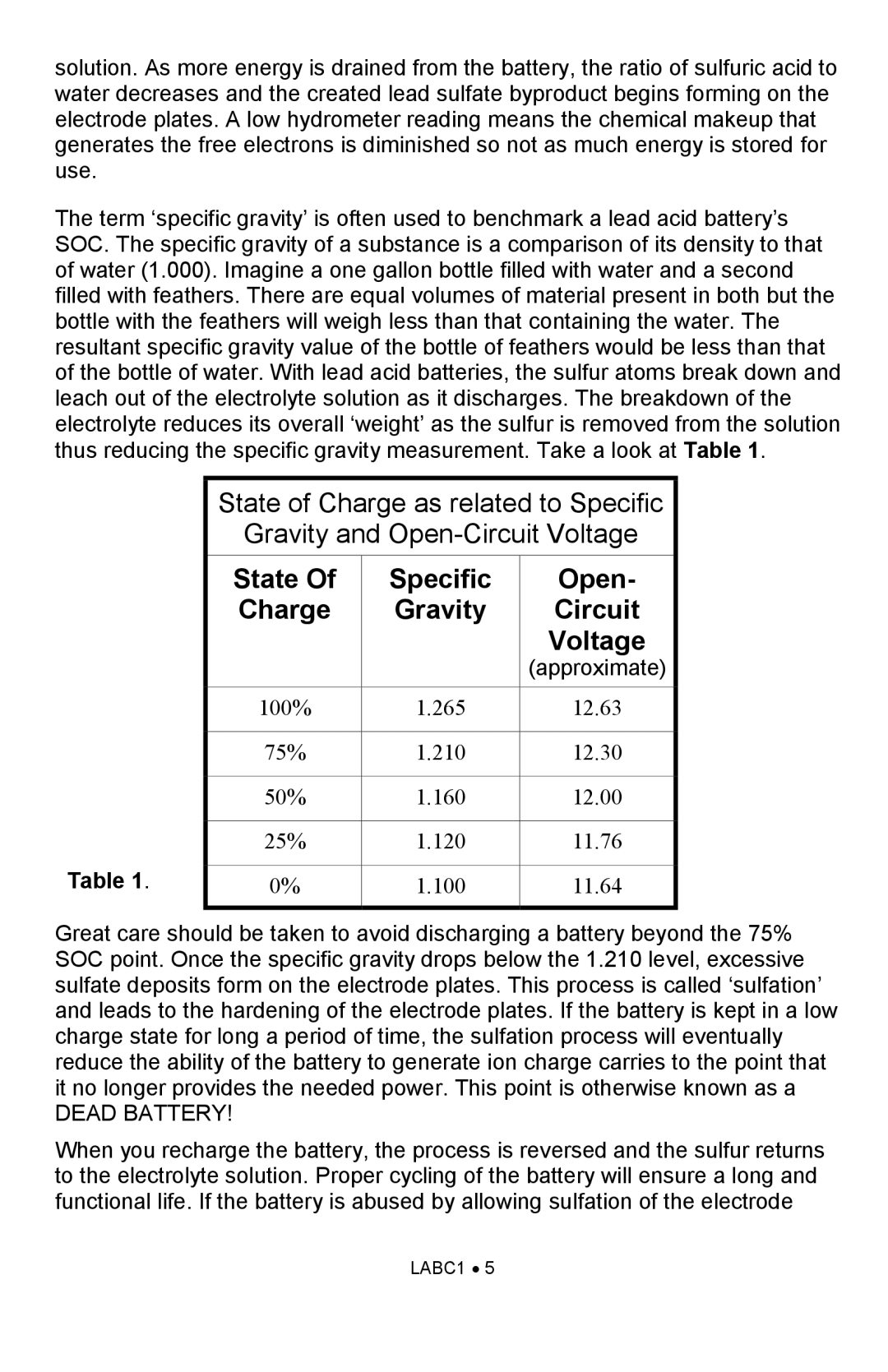
The term ‘specific gravity’ is often used to benchmark a lead acid battery’s SOC. The specific gravity of a substance is a comparison of its density to that of water (1.000). Imagine a one gallon bottle filled with water and a second filled with feathers. There are equal volumes of material present in both but the bottle with the feathers will weigh less than that containing the water. The resultant specific gravity value of the bottle of feathers would be less than that of the bottle of water. With lead acid batteries, the sulfur atoms break down and leach out of the electrolyte solution as it discharges. The breakdown of the electrolyte reduces its overall ‘weight’ as the sulfur is removed from the solution thus reducing the specific gravity measurement. Take a look at Table 1.
Table 1.
State of Charge as related to Specific
Gravity and
State Of | Specific | Open- |
Charge | Gravity | Circuit |
|
| Voltage |
|
| (approximate) |
100% | 1.265 | 12.63 |
|
|
|
75% | 1.210 | 12.30 |
|
|
|
50% | 1.160 | 12.00 |
|
|
|
25% | 1.120 | 11.76 |
|
|
|
0% | 1.100 | 11.64 |
|
|
|
Great care should be taken to avoid discharging a battery beyond the 75% SOC point. Once the specific gravity drops below the 1.210 level, excessive sulfate deposits form on the electrode plates. This process is called ‘sulfation’ and leads to the hardening of the electrode plates. If the battery is kept in a low charge state for long a period of time, the sulfation process will eventually reduce the ability of the battery to generate ion charge carries to the point that it no longer provides the needed power. This point is otherwise known as a
DEAD BATTERY!
When you recharge the battery, the process is reversed and the sulfur returns to the electrolyte solution. Proper cycling of the battery will ensure a long and functional life. If the battery is abused by allowing sulfation of the electrode
LABC1 • 5