User’s Guide
Page
Welcome
Motorola Atrix 4G
Your Phone
For keyboard details, see Text Entry on
Find More Information
On the web-You can also get support online
Contents
Let’s Go
Dialer Contacts, then
Set Up & Go
Your Motoblur Account
Security
Wi-Fi Connect
Set up email accounts, see Set Up Messaging on
To add or edit the email, social
Contacts
Touchscreen & Keys
Quick start Touchscreen
Dialer Contacts then drag your list up or down
Location & security Set up screen lock
Power & Sleep Key
Volume Keys
Display Screen timeout
Quick Start Home Screen
Home Screen
Menu, Home, Search, & Back Keys
Hold one until you
Messages, Social Networking, or Calendar widgets
Widgets, touch
Feel a vibration
Phone Status & Notifications
Personalize
Search
Quick Start Personalize
Sounds
Display Settings
Ringtones
Language & Region
Find it Menu Settings Language & keyboard Select locale
Apps
Quick start Apps
Recent Apps
Manage & Restore Apps
Find it Menu Settings Applications Manage applications
Market, touch My apps
Choose Carefully
Privacy, use apps from trusted sites, like
Quick Start Calls
Update Your Phone
Calls
Find it Dialer
Touch End call
Make & Answer Calls
End Calls
Your phone to your
Voice Dial
Speed Dial
Favorites
Handsfree
Conference Calls
Mute & Hold
Enter Numbers During a Call
Your Phone Number Recent Calls
Restrict Outgoing Calls
Settings Additional settings Caller ID
Call Forwarding & Waiting
Cool Down
Quick Start Contacts
Access
Contacts
Find it Dialer Contacts
Hold the contact, then choose Share name card
Transfer Contacts
Status
Touch Motorola widgets Mobile SMS Contact quick tasks
Edit or Delete Contacts
To add your email contacts, see Set Up Messaging on
Call, Text, or Email Contacts
Synchronize Contacts
Link Contacts
Create Contacts
My Details
Quick Start Social Networking
Social Networking
Groups
To edit details, touch Menu Edit
Find it Accounts Add account
Update Your Status
Add Accounts
Quick Start Messaging
Text Messaging & Email
Edit & Delete Accounts
Find it Accounts
Find it Messaging
Create Messages
Send & Receive Attachments
Accounts Add account
Set Up Messaging
To add email accounts, touch
Accounts show messages
Sends and receives messages Fetch schedule
Instant Messages
Voicemail
Messaging Menu , then
Text Entry
Quick Start Text Entry
Text Entry Settings
Swype
Photos & Videos
Settings Video Resolution Medium Qvga
To zoom in or out, press the volume keys
Quick Start Photos & Videos
Share
View & Share Your Photos & Videos
Find it Gallery
Photo & Video Settings
Options
Quick Start Music
Music
YouTube
Find it YouTube
To load and play music on your phone, you need a
Airplane mode
Get Music
To transfer music files from your computer to your phone
Connect
Browser
Quick Start Browser
More Downloads
Download Apps
To download apps
To clear your download history
Find it Maps
Location
Quick Start Location
Help
Find it Navigation
Google Maps Navigation
Google Latitude
Follow the prompts to speak or type your destination
Settings Do not detect your location
AT&T Features & Services
Features & Services
More Information
Webtop Application & Entertainment Center
Quick start Webtop Application & Entertainment Center
Webtop Application
Choose webtop
Entertainment Center
Music stored on your phone Touch
Quick Start Bluetooth & Wi-Fi Connections
Touch Menu Settings Wireless & networks Wi-Fi settings
Bluetooth Handsfree Devices
After you connect
Disconnect
Wi-Fi Networks
Bluetooth devices list
To connect a network in your range
To set up your phone as a Wi-Fi hotspot
Dlna Media Devices
File Transfer
Airplane Mode
Use airplane mode to turn all your
Quick Start File Transfer
To remove or format your memory Card, you need to unmount it
Then, to format your memory card, touch Format SD card
To use your
Phone Portal
Touch Phone Portal
Connection
Find it Quickoffice
Quickoffice
Use Quickoffice to view and edit files on your memory card
Find it Calendar
Schedule
Quick Start Schedule
Delete event
Quick Start Security
Alarm Clock
Security
Date & Time
Change SIM PIN
Reset
Security Set up SIM card lock
To wipe your phone data
Lost, Stolen, Broken, Cleared
To locate your phone
Tips & Tricks
Battery Tips
Touch Menu Settings Wireless
Location & security Use GPS satellites
Settings Display Screen timeout
Accessibility
Settings Display Brightness dimmer setting
Settings Accessibility. Touch
Find it Menu Settings Sound Volume or Vibrate
Call settings Caller ID Readout
Find it Voice Commands Menu Settings
Tip To set separate volumes for calls
Sound
Around the screen Touch Menu Settings Accessibility. Touch
Accessibility to enable the settings, then touch Zoom Mode
TTY
Connections Quick Reference
Apps
Service & Repairs
Troubleshooting
Crash Recovery
Safety, Regulatory & Legal
Battery Use & Safety
Battery Charging
Third Party Accessories Driving Precautions
Seizures/Blackouts
While driving, Always
Glass Parts
Repetitive Motion
Children
Operational Warnings
Radio Frequency RF Energy
Authorities for more information
Specific Absorption Rate Ieee
SAR
Information from the World Health Organization
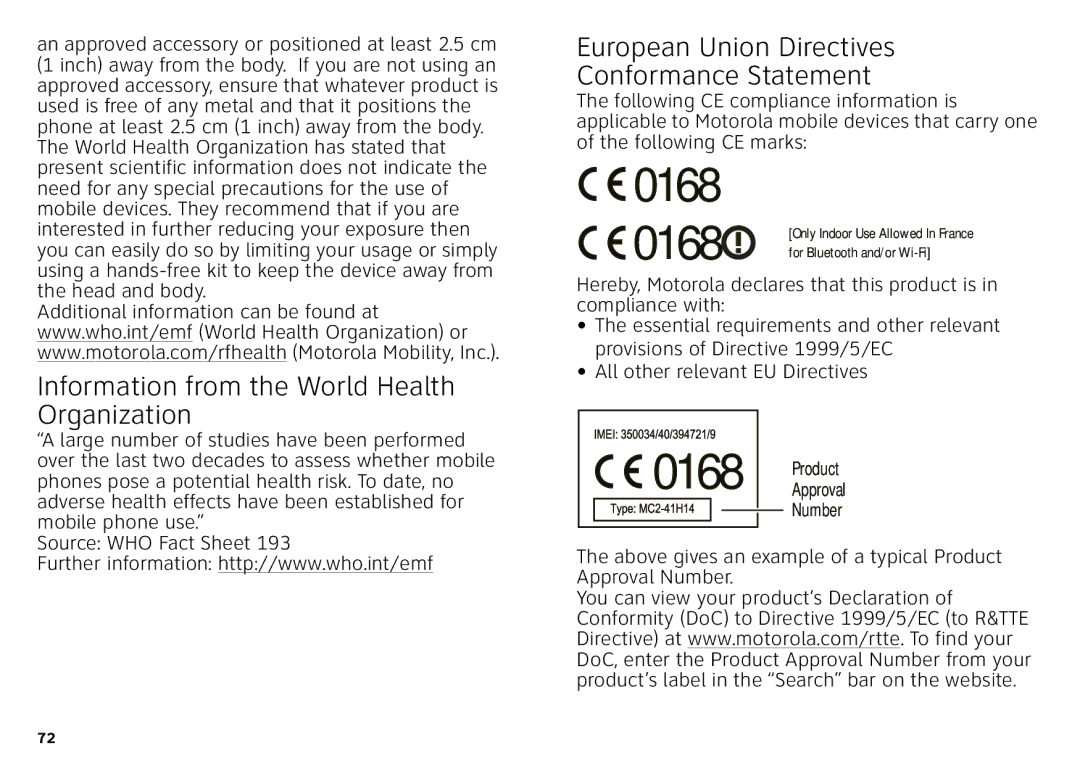
European Union Directives Conformance Statement
FCC Notice to Users
Industry Canada Notice to Users
Location Services GPS & Agps
Navigation
Smart Practices While Driving
Privacy & Data Security
Use & Care
Liquids
Mobile Devices & Accessories Packaging & Product Guides
Recycling
Open Source Software Information
Content Copyright
Software Copyright Notice
Product Registration
Service & Repairs
Export Law Assurances
Motorola Limited Warranty for the United States and Canada
What Does this Warranty Cover?
Who is Covered?
How to Obtain Warranty Service or Other Information
Copyright & Trademarks

![]()
![]() 0168
0168