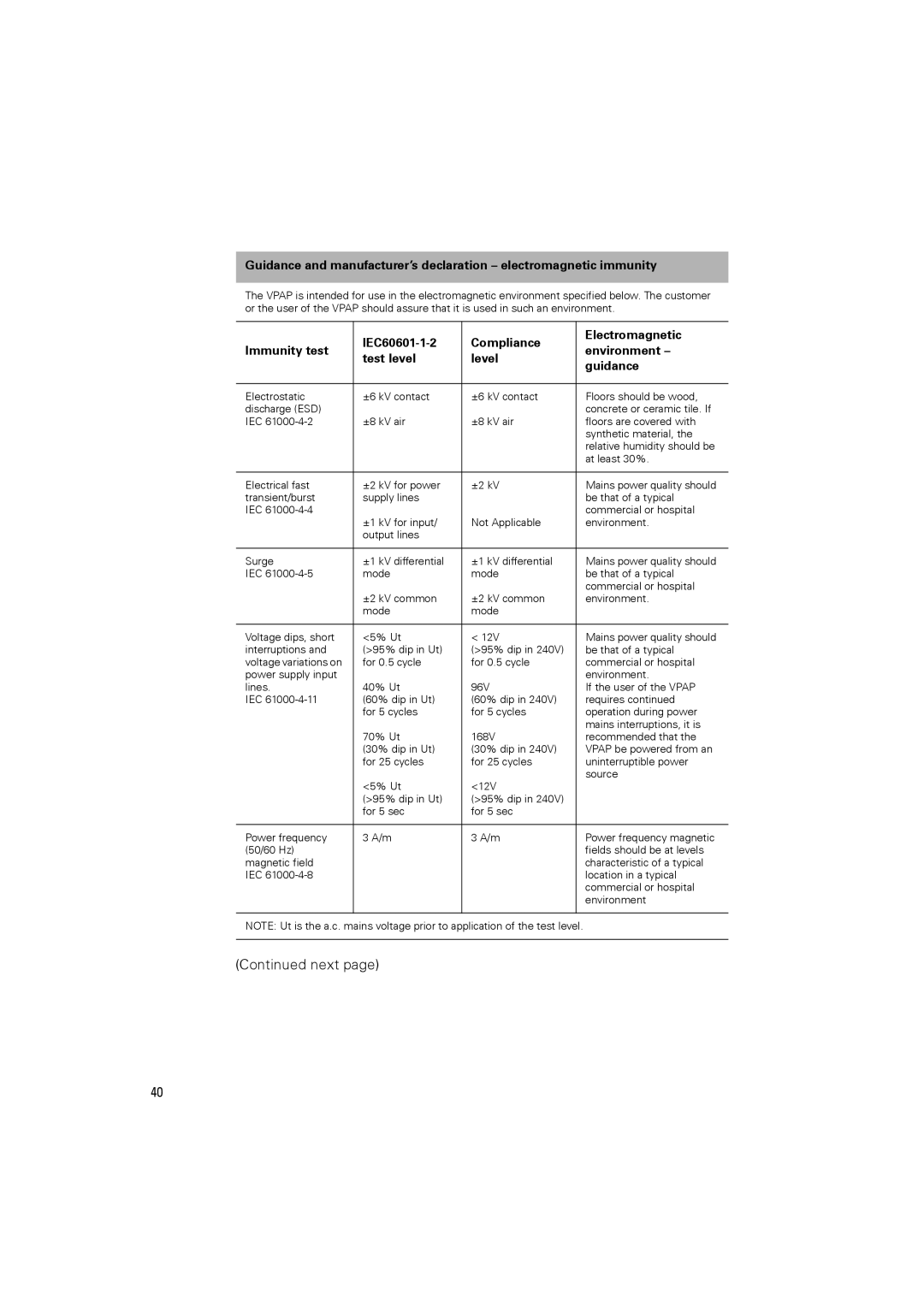
248127 specifications
ResMed 248127 is a state-of-the-art device designed to enhance patients' experience in managing sleep apnea and other respiratory conditions. As a leader in innovative technologies, ResMed continues to focus on developing equipment that promotes better sleep and overall health.One of the key features of the ResMed 248127 is its advanced integrated monitoring capabilities. This device provides real-time data on various parameters that are crucial for effective therapy management. Patients and healthcare providers can easily access important information, such as adherence rates and overall treatment effectiveness. This transparency helps to ensure that users remain compliant with their prescribed therapies, which is essential for achieving desired outcomes.
The ResMed 248127 also features a user-friendly interface with an intuitive design. The device is equipped with a high-resolution display that presents information clearly, making it easier for users to navigate through different settings and options. This simplicity is particularly beneficial for older adults or those who may not be technologically savvy, allowing for an efficient user experience without overwhelming the patient.
Another standout characteristic is its whisper-quiet operation. Sleep therapy devices often come under scrutiny for their noise levels, which can disrupt sleep patterns. The ResMed 248127 addresses this concern by utilizing advanced sound dampening technologies that ensure a peaceful night's sleep, creating a harmonious environment for both the patient and their sleep partner.
The device also incorporates SmartStart technology, which automatically starts or stops therapy as soon as the mask is put on or removed. This functionality not only enhances the convenience of use but also ensures that therapy is delivered effectively and promptly as needed.
Furthermore, the ResMed 248127 is designed with portability in mind. Its lightweight and compact design make it easy for users to transport the device, facilitating therapy adherence even while traveling. This flexibility allows patients to maintain their treatment regimen when they are away from home, ensuring that sleep quality is preserved regardless of location.
In conclusion, the ResMed 248127 combines innovative technology, user-friendly features, and unmatched portability to provide users with an effective solution for managing sleep apnea and other respiratory conditions. Its advanced monitoring capabilities, quiet operation, and convenience-oriented design make it a valuable tool in the quest for better sleep health. This device demonstrates ResMed's commitment to improving patient outcomes through technological advancements in the realm of sleep therapy.