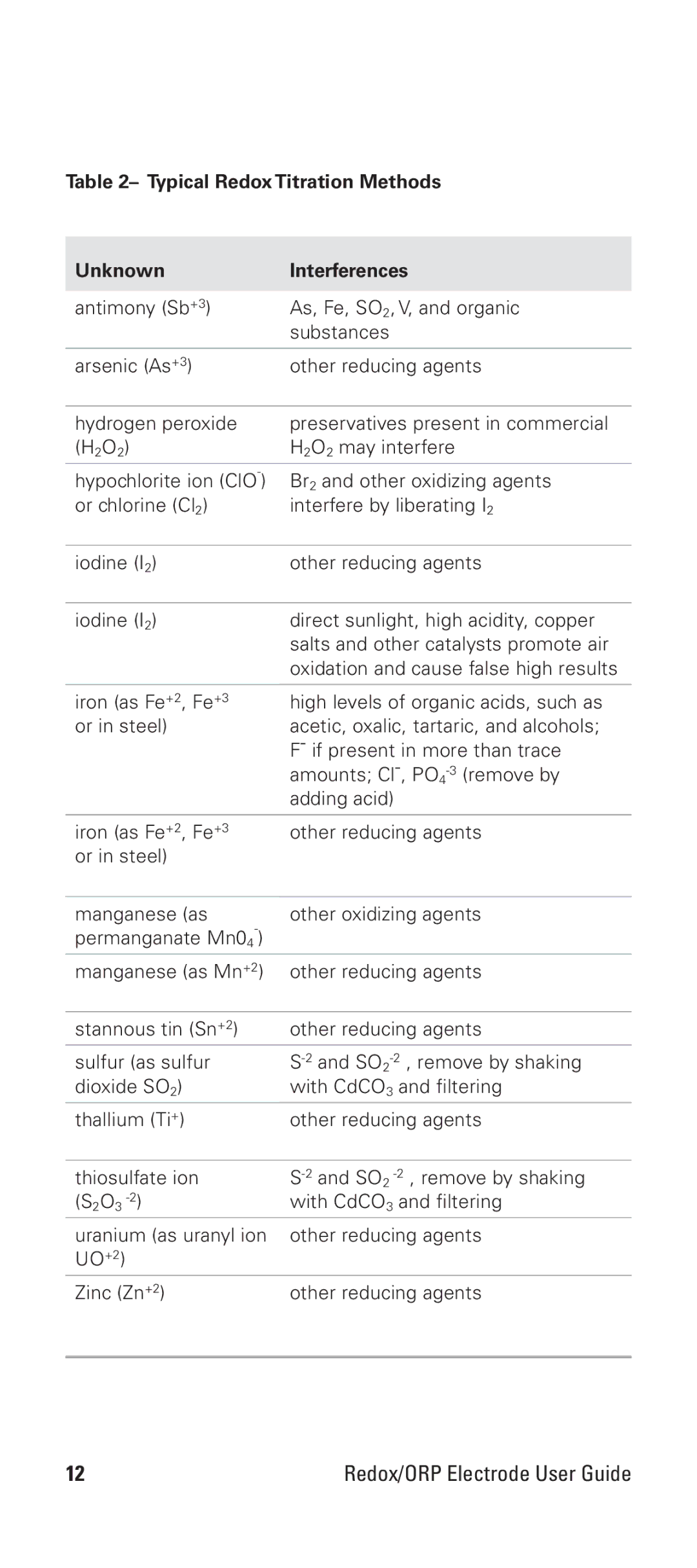
Table 2– Typical Redox Titration Methods
Unknown | Interferences |
antimony (Sb+3) | As, Fe, SO2, V, and organic |
| substances |
|
|
arsenic (As+3) | other reducing agents |
|
|
hydrogen peroxide | preservatives present in commercial |
(H2O2) | H2O2 may interfere |
hypochlorite ion (ClO¯) | Br2 and other oxidizing agents |
or chlorine (Cl2) | interfere by liberating I2 |
|
|
iodine (I2) | other reducing agents |
|
|
iodine (I2) | direct sunlight, high acidity, copper |
| salts and other catalysts promote air |
| oxidation and cause false high results |
|
|
iron (as Fe+2, Fe+3 | high levels of organic acids, such as |
or in steel) | acetic, oxalic, tartaric, and alcohols; |
| F¯ if present in more than trace |
| amounts; Cl¯, |
| adding acid) |
|
|
iron (as Fe+2, Fe+3 | other reducing agents |
or in steel) |
|
|
|
manganese (as | other oxidizing agents |
permanganate Mn04¯) |
|
manganese (as Mn+2) | other reducing agents |
|
|
stannous tin (Sn+2) | other reducing agents |
sulfur (as sulfur | |
dioxide SO2) | with CdCO3 and filtering |
thallium (Ti+) | other reducing agents |
|
|
thiosulfate ion | |
(S2O3 | with CdCO3 and filtering |
uranium (as uranyl ion | other reducing agents |
UO+2) |
|
Zinc (Zn+2) | other reducing agents |
|
|
12 | Redox/ORP Electrode User Guide |