English
Technical data
This device fulfills the provisions of the EC directive 93/42/EEC (Medical Device Directive) and the European Standard
Type: | OMRON CompAIR CXpro | |
Dimensions: | 160 (W) x 90 (H) x 220 (D) mm. | |
Weight: | approx. 2 kg |
|
Nebulisation rate: | 0.3* ml/min average | |
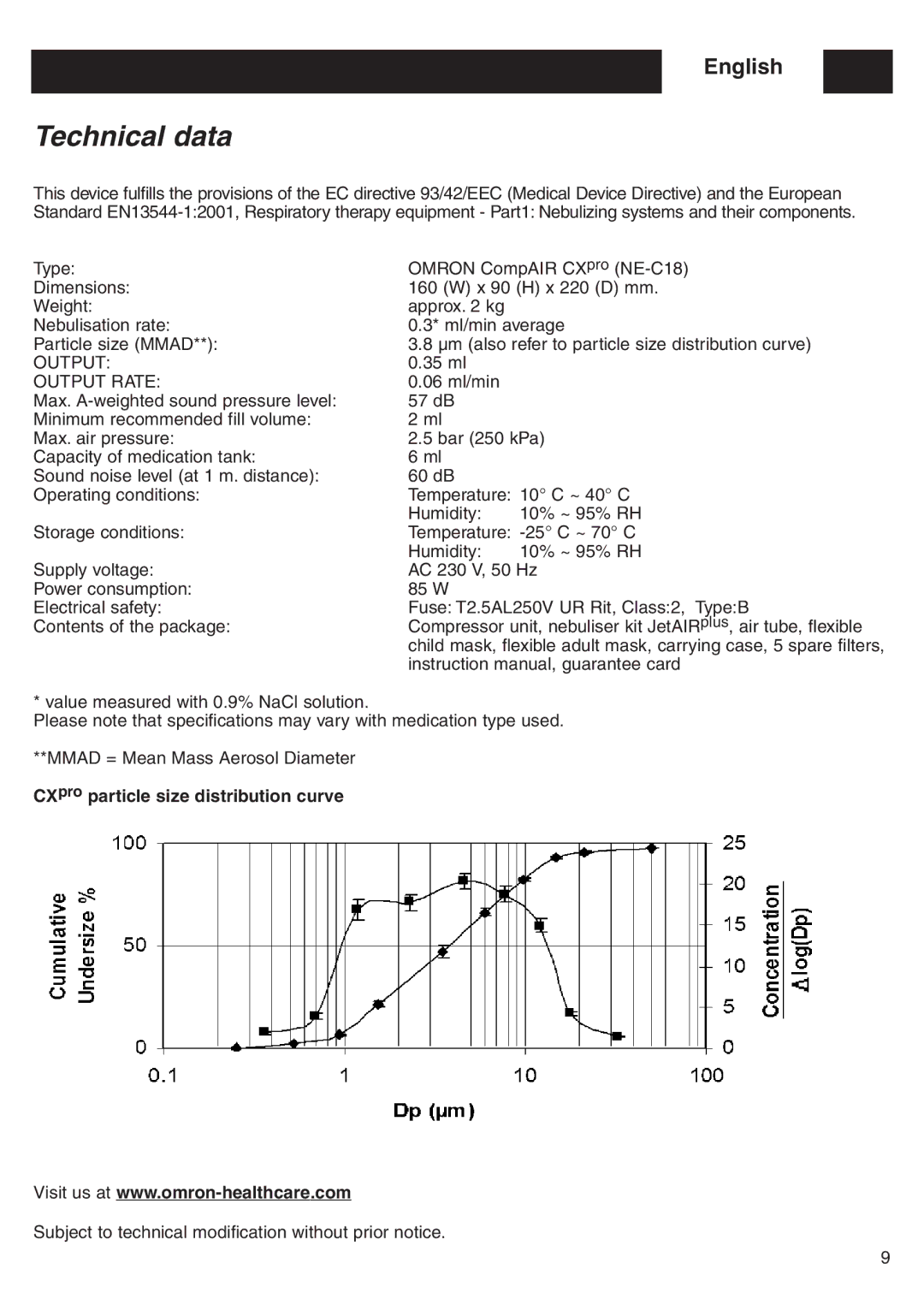
Particle size (MMAD**): | 3.8 µm (also refer to particle size distribution curve) | |
OUTPUT: | 0.35 ml |
|
OUTPUT RATE: | 0.06 ml/min |
|
Max. | 57 dB |
|
Minimum recommended fill volume: | 2 ml |
|
Max. air pressure: | 2.5 bar (250 kPa) | |
Capacity of medication tank: | 6 ml |
|
Sound noise level (at 1 m. distance): | 60 dB |
|
Operating conditions: | Temperature: 10° C ~ 40° C | |
| Humidity: | 10% ~ 95% RH |
Storage conditions: | Temperature: | |
| Humidity: | 10% ~ 95% RH |
Supply voltage: | AC 230 V, 50 Hz | |
Power consumption: | 85 W |
|
Electrical safety: | Fuse: T2.5AL250V UR Rit, Class:2, Type:B | |
Contents of the package: | Compressor unit, nebuliser kit JetAIRplus, air tube, flexible | |
child mask, flexible adult mask, carrying case, 5 spare filters, instruction manual, guarantee card
* value measured with 0.9% NaCl solution.
Please note that specifications may vary with medication type used.
**MMAD = Mean Mass Aerosol Diameter
CXpro particle size distribution curve
Visit us at
Subject to technical modification without prior notice.
9