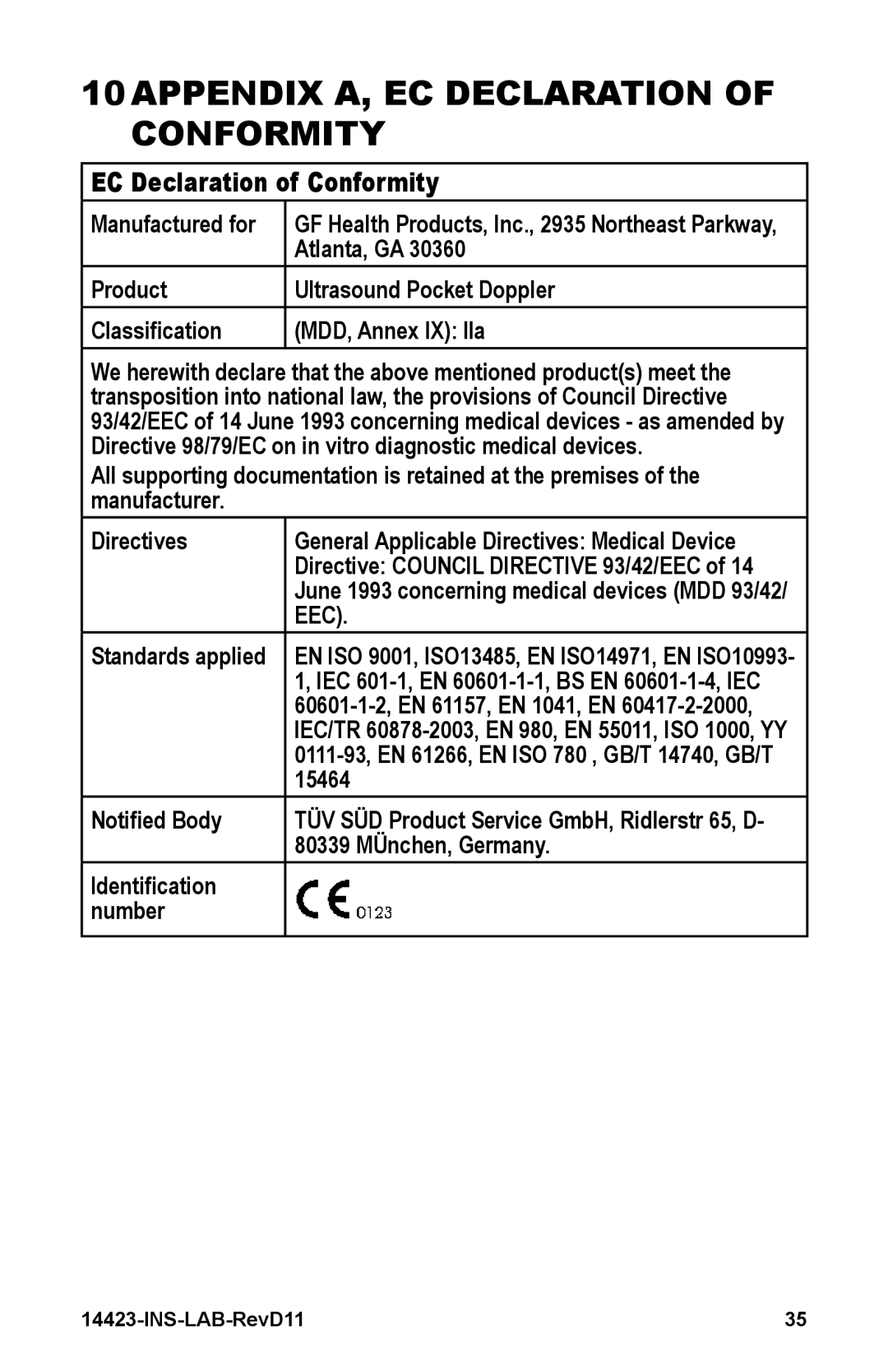
10 APPENDIX A, EC DECLARATION OF CONFORMITY
EC Declaration of Conformity
Manufactured for | GF Health Products, Inc., 2935 Northeast Parkway, |
| Atlanta, GA 30360 |
|
|
Product | Ultrasound Pocket Doppler |
|
|
Classification | (MDD, Annex IX): IIa |
We herewith declare that the above mentioned product(s) meet the transposition into national law, the provisions of Council Directive 93/42/EEC of 14 June 1993 concerning medical devices - as amended by Directive 98/79/EC on in vitro diagnostic medical devices.
All supporting documentation is retained at the premises of the manufacturer.
Directives | General Applicable Directives: Medical Device |
| Directive: COUNCIL DIRECTIVE 93/42/EEC of 14 |
| June 1993 concerning medical devices (MDD 93/42/ |
| EEC). |
Standards applied | EN ISO 9001, ISO13485, EN ISO14971, EN ISO10993- |
| 1, IEC |
| |
| IEC/TR |
| |
| 15464 |
Notified Body | TÜV SÜD Product Service GmbH, Ridlerstr 65, D- |
| 80339 MÜnchen, Germany. |
Identification |
|
number |
|
|
|
| 35 |