2 Operational Theory | Model 3300PA | |
|
|
|
|
|
|
|
|
|
If the pressure changes, the rate that oxygen reaches the cathode through the diffusing membrane will also increase. The electron transfer, and therefore the external current, will increase, even though the proportion of oxygen has not changed.
Fortunately, Dalton's Law confirms that every gas in a mixture contributes the same pressure to the mixture that it would exert if it were alone in the same amount in that same volume. This means that as long as the total pressure of the sample remains constant, the mixture can change, but the diffusion of the oxygen will be affected only by the concentration of the oxygen.
For this reason, the sample system supplying sample gas to the cell should be designed to keep the pressure on the diffusion membrane constant.
2.2.5 Calibration Characteristics
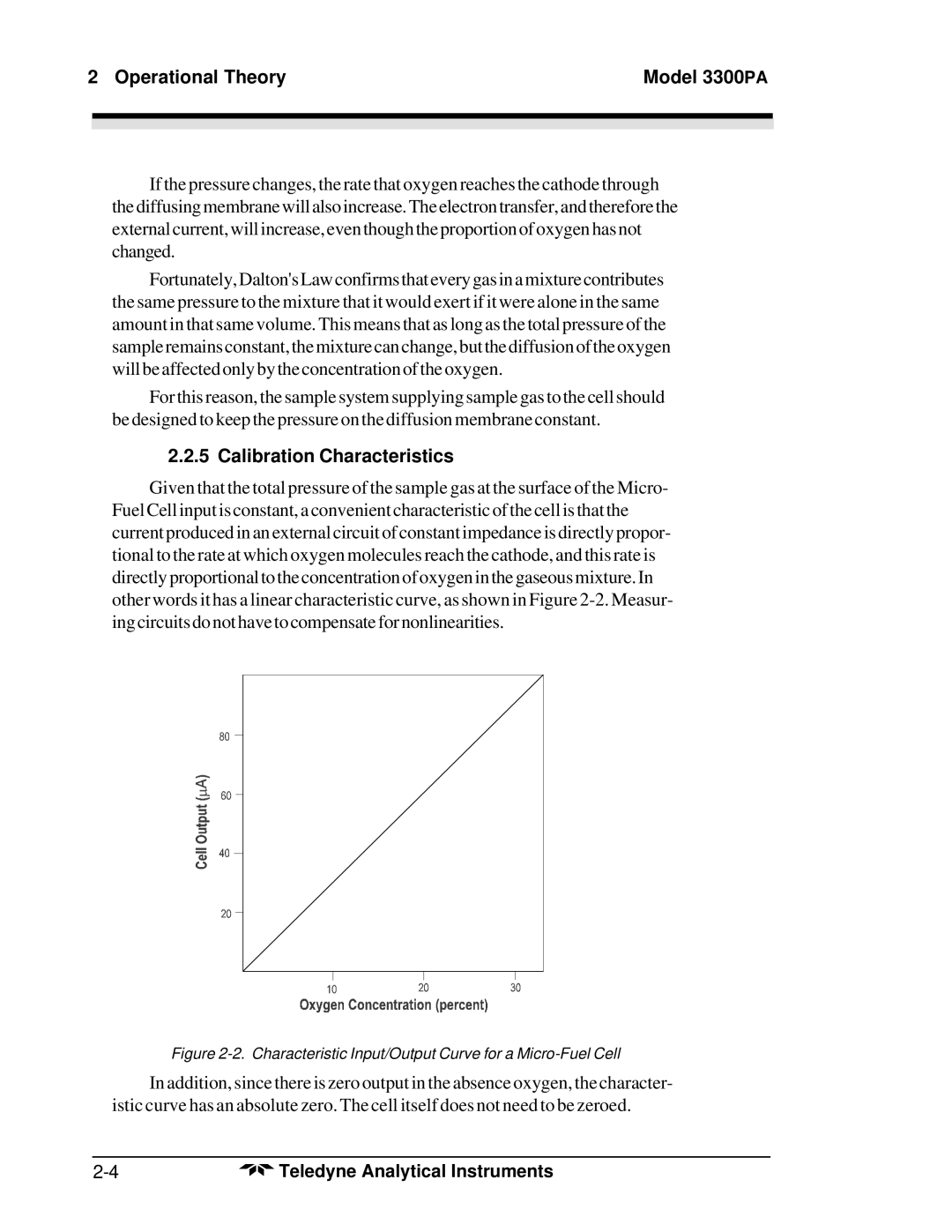
Given that the total pressure of the sample gas at the surface of the Micro- Fuel Cell input is constant, a convenient characteristic of the cell is that the current produced in an external circuit of constant impedance is directly propor- tional to the rate at which oxygen molecules reach the cathode, and this rate is directly proportional to the concentration of oxygen in the gaseous mixture. In other words it has a linear characteristic curve, as shown in Figure
Figure 2-2. Characteristic Input/Output Curve for a Micro-Fuel Cell
In addition, since there is zero output in the absence oxygen, the character- istic curve has an absolute zero. The cell itself does not need to be zeroed.
Teledyne Analytical Instruments |