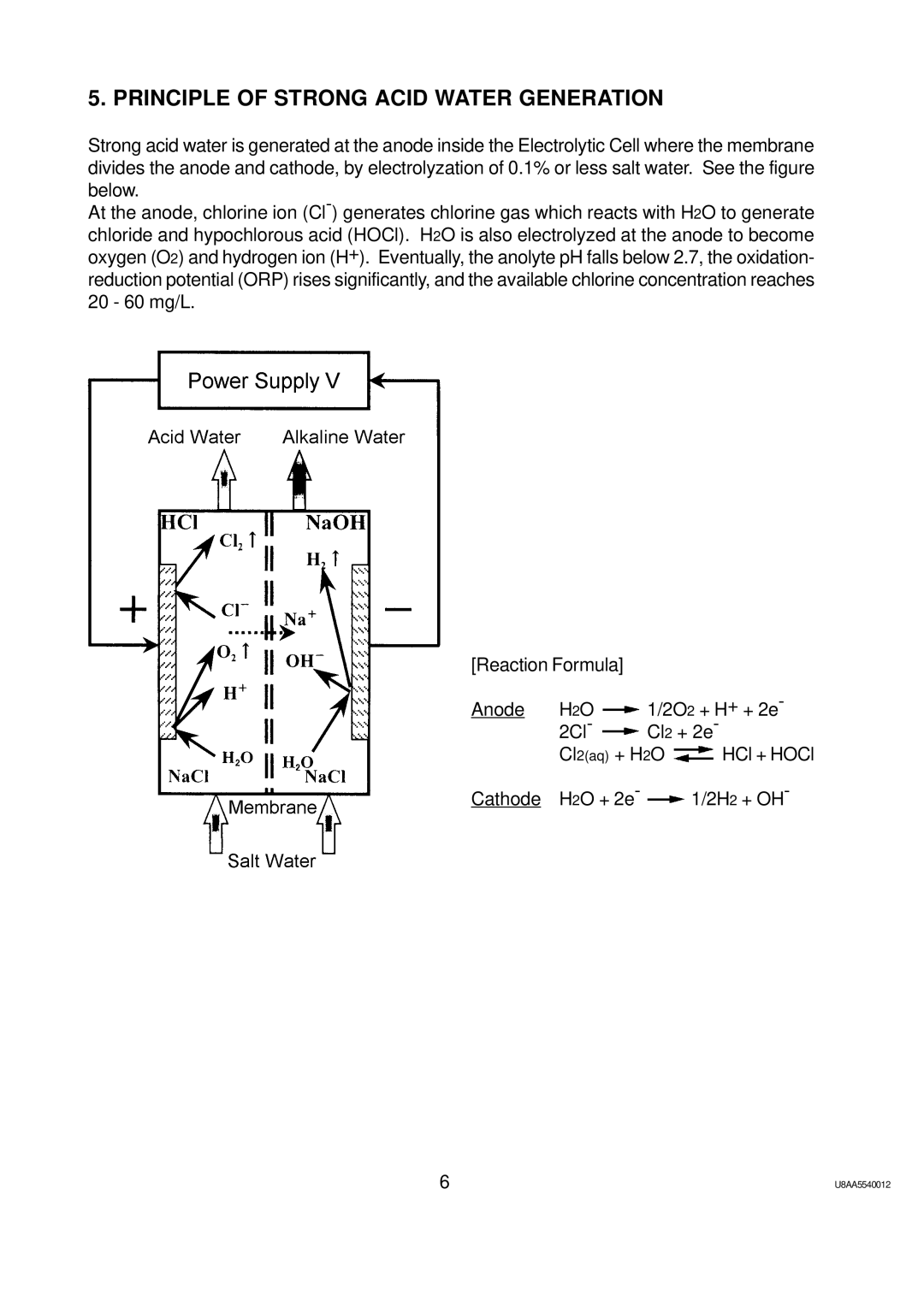
5. PRINCIPLE OF STRONG ACID WATER GENERATION
Strong acid water is generated at the anode inside the Electrolytic Cell where the membrane divides the anode and cathode, by electrolyzation of 0.1% or less salt water. See the figure below.
At the anode, chlorine ion
[Reaction Formula] |
|
| |
Anode | H2O | 1/2O2 + H+ + 2e- | |
| 2Cl- | Cl2 + 2e- | |
| Cl2(aq) + H2O | HCl + HOCl | |
Cathode | H2O + 2e- |
| 1/2H2 + OH- |
6 | U8AA5540012 |