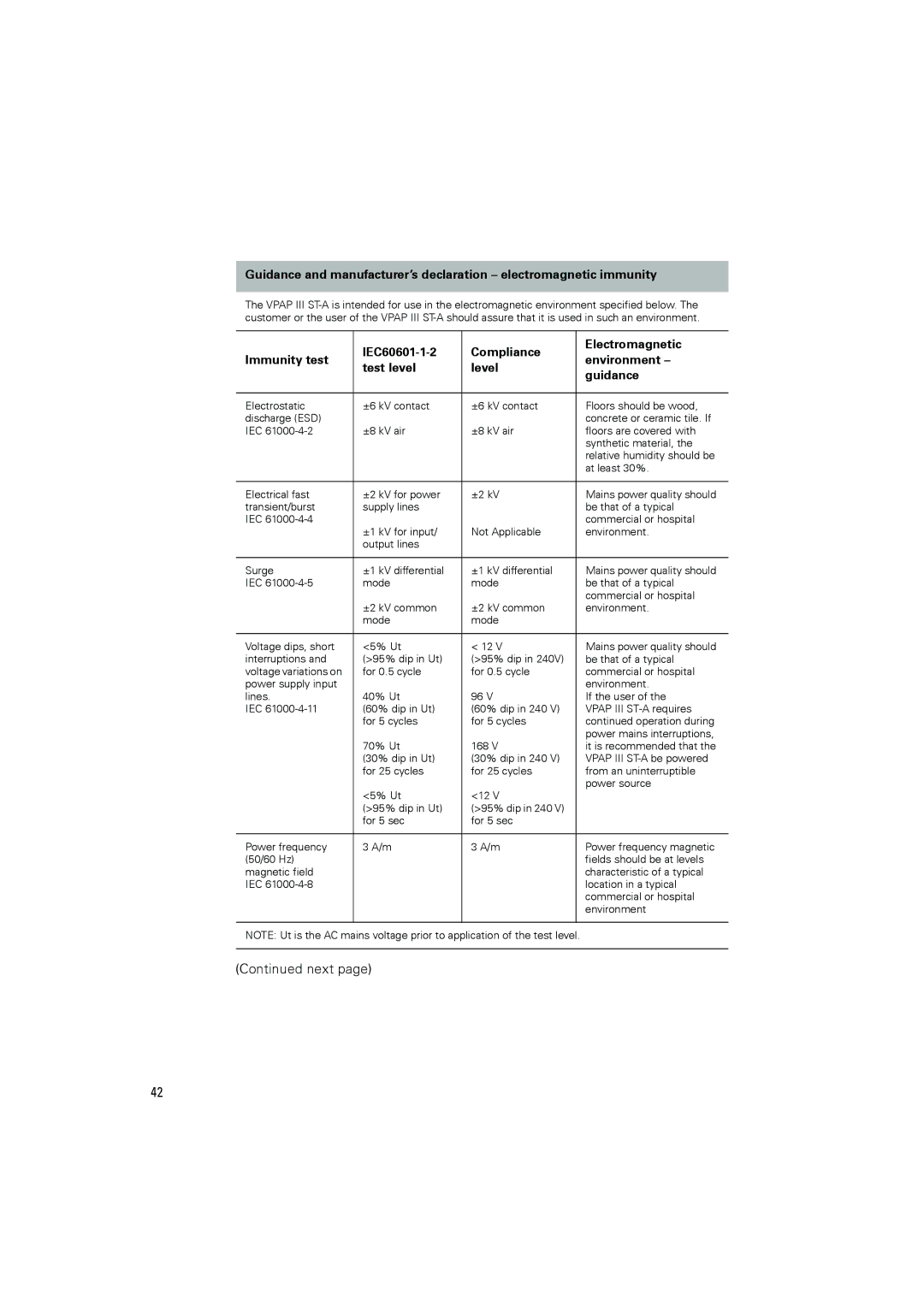
III ST-A specifications
The ResMed III ST-A is a state-of-the-art ventilator specifically designed for patients with respiratory challenges, particularly those suffering from obstructive sleep apnea, neuromuscular conditions, or chronic obstructive pulmonary disease (COPD). It stands out due to its user-friendly features, advanced technologies, and robust performance, making it a preferred choice for both clinicians and patients.One of the main features of the ResMed III ST-A is its intelligent algorithm designed to optimize minute ventilation. This provides variable support that adapts to the patient's breathing patterns, enhancing comfort and ensuring effective ventilation. By offering a range of modes, including spontaneous timed (ST) and volume-assured pressure support (VAPS), this device caters to various clinical needs, facilitating customized therapy based on individual patient requirements.
The built-in leak compensation feature of the ResMed III ST-A allows it to maintain accurate pressure delivery even in the presence of large leaks, which is crucial for ensuring effective therapy. This is particularly beneficial for patients who may have difficulty achieving a proper seal with their masks.
Incorporating advanced technologies, the ResMed III ST-A features AutoSet technology, which automatically adjusts pressure levels in response to detected changes in airflow. This promotes optimal treatment at all times, reducing the burden on patients and allowing for a more seamless sleep experience. The device also supports a range of connectivity options, enabling healthcare providers to monitor patient compliance and therapy effectiveness remotely through ResMed's cloud-based platform.
The ResMed III ST-A is designed with a user-friendly interface, including a bright, easy-to-read display and simplified controls, promoting ease of operation for both healthcare professionals and patients. Its compact size and lightweight design enhance portability, making it suitable for home and travel use.
Furthermore, the device's comprehensive data logging capabilities allow clinicians to access a wealth of information regarding patient adherence and sleep patterns, thus supporting informed decision-making. The ResMed III ST-A also prioritizes patient safety, featuring alarms and alerts for high- and low-pressure conditions.
In conclusion, the ResMed III ST-A embodies cutting-edge technology and thoughtful design, providing versatile and effective respiratory support. With its combination of intelligent algorithms, user-friendly features, and robust patient monitoring capabilities, it is well-positioned to enhance the quality of life for individuals with respiratory challenges.