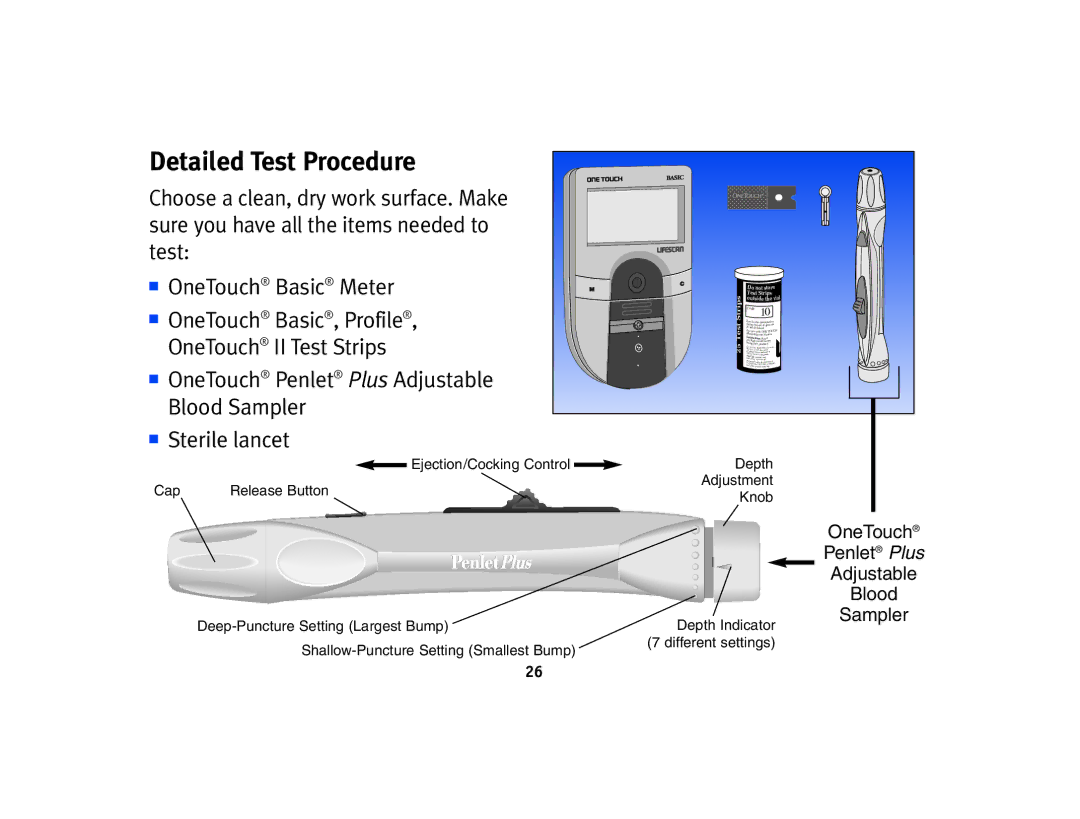
Detailed Test Procedure |
|
Choose a clean, dry work surface. Make |
|
sure you have all the items needed to |
|
test: |
|
■ OneTouch® Basic® Meter | M |
■ OneTouch® Basic®, Profile®, |
|
OneTouch® II Test Strips |
|
■ OneTouch® Penlet® Plus Adjustable |
|
Blood Sampler |
|
| |
■ Sterile lancet |
|
C | Do not | store | |
| Test Strips | ||
Strips | outsi de t he vial | ||
Code | 10 | ||
|
| ||
Test | Test for th equ antitative | ||
measurem ent | o fglucose | ||
package in sert | before | ||
| in whole b loo d . | ||
| For use wi | th ONE TOUCH | |
| Blood Blu cose Meters. | ||
| Instructio ns: R ead | ||
25 | using this | pro duct. | |
a(3t 0te˚Cm)p. Deroatnuort esreufrnidg rerea8te6.˚F | |||
| For in vitro di | agno s tci use only. | |
| Store in a coo | l,dry | place |
| Vial Cap: con | tains | not |
| more than 3g | silica | gel |
| Protected und er th e ofllowing | ||
| U.S. Patents 4 | ,935, | 3 64, 5,049,487, |
| and other pate | nts p ending. | |
| Ejection/Cocking Control |
Cap | Release Button |
Depth
Adjustment
Knob
Depth Indicator (7 different settings)
OneTouch®
Penlet® Plus
Adjustable
Blood
Sampler
26