period one
Absorption Refrigeration Cycle
notes
|
|
|
|
|
|
|
|
|
|
|
|
|
| 15 psia |
|
| concentration |
|
|
|
|
|
|
|
|
|
|
|
|
|
| [103.4 kPa] |
| 0 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| e |
|
|
| 0 |
| |
|
|
|
|
|
|
|
|
|
| r |
|
|
|
| |||
|
|
|
|
|
|
|
|
| u |
|
|
|
| 5 |
| ||
|
|
|
|
|
|
|
| s |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
| s |
|
|
| 5 psia |
|
|
| 5 |
| |
|
|
|
|
|
| re |
|
|
|
|
|
|
|
| |||
|
|
|
|
| p |
|
|
|
|
|
|
|
|
| 5 |
| |
|
|
|
| r |
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
| o |
|
|
|
|
|
|
| [34.5 kPa] |
|
|
|
|
| |
|
| p |
|
|
|
|
|
|
|
|
|
|
|
|
| ||
| a |
|
|
|
|
|
|
|
|
|
|
|
|
| 0 |
| |
| v |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| 1 psia |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| [6.9 kPa] |
|
|
|
|
|
|
|
|
|
|
| $ |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
0.1 psia |
|
|
|
|
|
| & |
|
| % |
|
|
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
[0.69 kPa] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 50°F |
|
|
|
|
|
|
|
|
|
| 100°F |
| 150°F | 200°F | LiBr solution | |
| [10°C] |
|
|
|
|
|
|
|
|
|
| [37.8°C] |
| [65.6°C] | [93.3°C] |
| Figure 18 |
|
|
|
|
|
|
|
|
|
|
|
| solution temperature |
|
| |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||
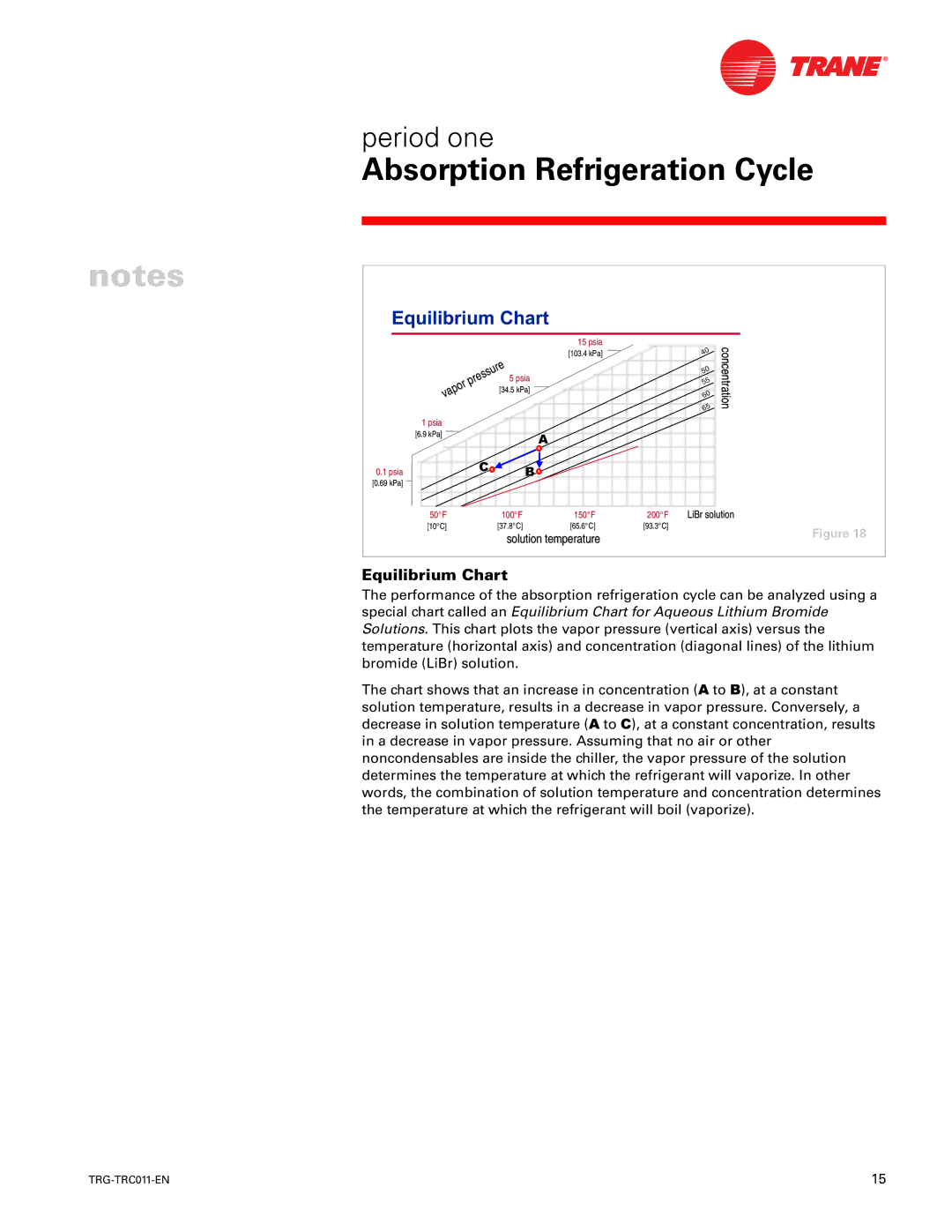
Equilibrium Chart
The performance of the absorption refrigeration cycle can be analyzed using a special chart called an Equilibrium Chart for Aqueous Lithium Bromide Solutions. This chart plots the vapor pressure (vertical axis) versus the temperature (horizontal axis) and concentration (diagonal lines) of the lithium bromide (LiBr) solution.
The chart shows that an increase in concentration ($ to %), at a constant solution temperature, results in a decrease in vapor pressure. Conversely, a decrease in solution temperature ($ to &), at a constant concentration, results in a decrease in vapor pressure. Assuming that no air or other noncondensables are inside the chiller, the vapor pressure of the solution determines the temperature at which the refrigerant will vaporize. In other words, the combination of solution temperature and concentration determines the temperature at which the refrigerant will boil (vaporize).
15 |