period three
Capacity Control
notes
|
|
|
|
|
|
|
|
| 15 psia |
| 5 | concentration |
|
|
|
|
|
|
|
|
| [103.4 kPa] |
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| e |
| 0 |
|
|
|
|
|
|
|
|
|
| r |
|
| |
|
|
|
|
|
|
|
| u |
| 5 |
| |
|
|
|
|
|
|
| s |
|
|
|
| |
|
|
|
|
|
| s |
| 5 psia |
| 5 |
| |
|
|
|
|
| re |
|
|
|
| |||
|
|
|
| p |
|
|
|
|
| 5 |
| |
|
|
| r |
|
|
|
|
|
|
|
| |
|
| o |
|
|
|
|
| [34.5 kPa] |
|
|
| |
| p |
|
|
|
|
|
|
|
|
| ||
a |
|
|
|
|
|
|
|
|
| 0 |
| |
v |
|
|
|
|
|
|
|
|
| & |
| |
|
|
|
|
|
|
|
|
| % | 6 |
| |
1 psia |
|
|
|
|
|
|
|
|
| ' |
| |
|
|
|
|
|
|
|
|
|
|
|
| |
[6.9 kPa] |
|
|
|
|
|
|
|
|
|
|
|
|
0.1 psia | $ |
|
|
|
|
|
|
| crystallization |
| |
[0.69 kPa] | ) | ( | line |
|
| ||||||
50°F | 100°F |
|
|
| 150°F | 200°F | LiBr solution | ||||
[10°C] | [37.8°C] |
|
|
| [65.6°C] | [93.3°C] | Figure 45 | ||||
| solution temperature |
| |||||||||
|
|
| |||||||||
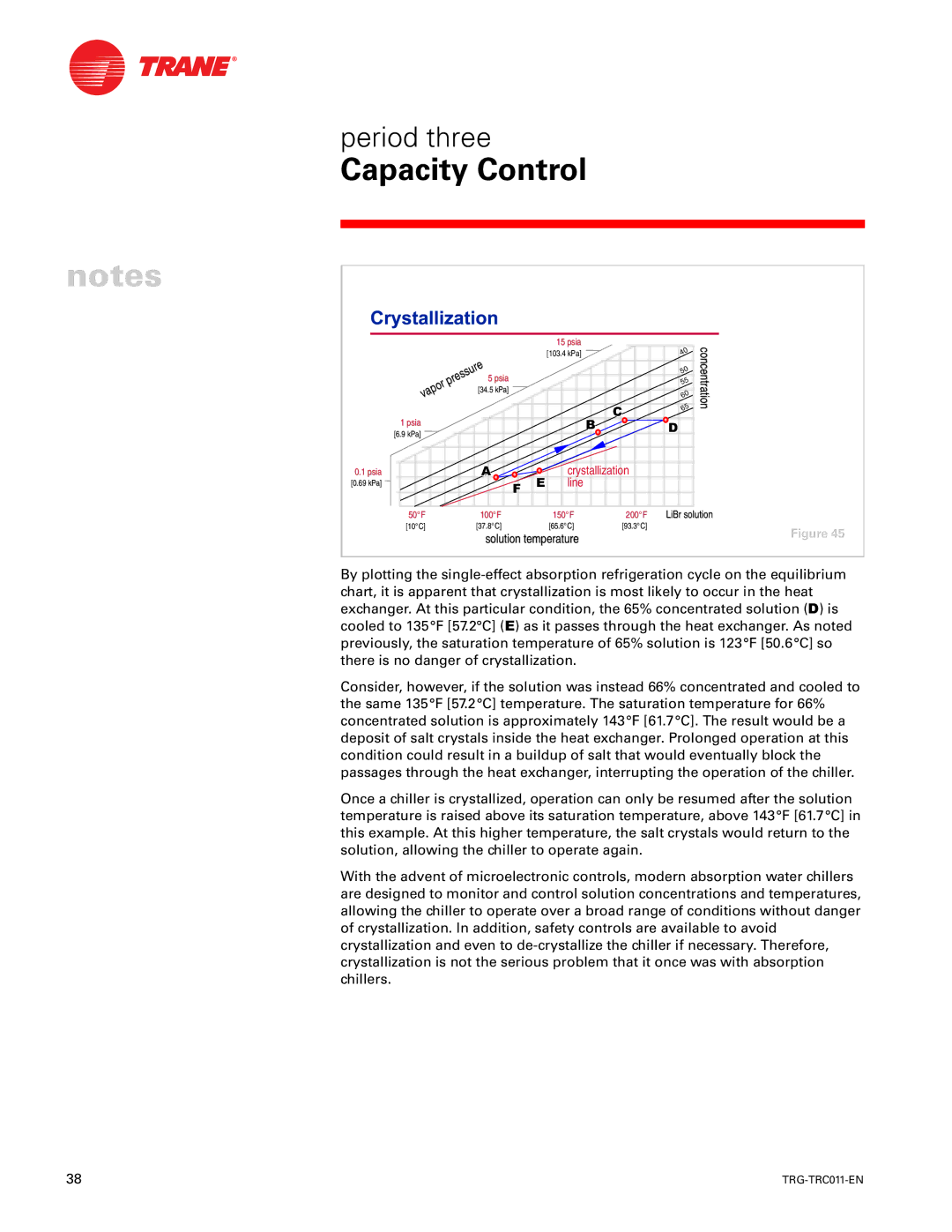
By plotting the
Consider, however, if the solution was instead 66% concentrated and cooled to the same 135°F [57.2°C] temperature. The saturation temperature for 66% concentrated solution is approximately 143°F [61.7°C]. The result would be a deposit of salt crystals inside the heat exchanger. Prolonged operation at this condition could result in a buildup of salt that would eventually block the passages through the heat exchanger, interrupting the operation of the chiller.
Once a chiller is crystallized, operation can only be resumed after the solution temperature is raised above its saturation temperature, above 143°F [61.7°C] in this example. At this higher temperature, the salt crystals would return to the solution, allowing the chiller to operate again.
With the advent of microelectronic controls, modern absorption water chillers are designed to monitor and control solution concentrations and temperatures, allowing the chiller to operate over a broad range of conditions without danger of crystallization. In addition, safety controls are available to avoid crystallization and even to
38 |
|