period one
Absorption Refrigeration Cycle
notes | The operating conditions used in this section of the clinic are approximate, |
subject to variation with changing load and | |
| conditions. |
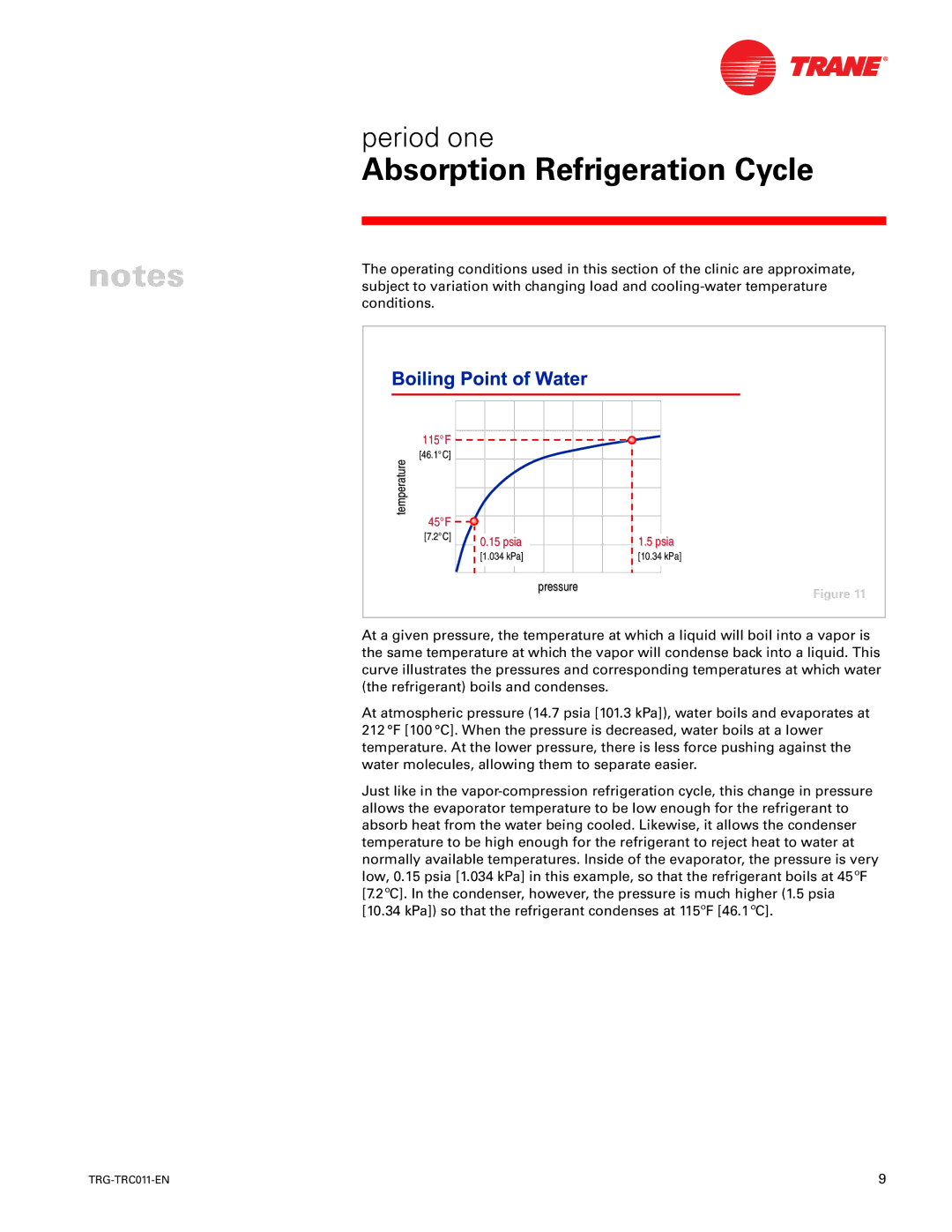
115°F |
|
|
|
[46.1°C] |
|
|
|
temperature |
|
|
|
45°F |
|
|
|
[7.2°C] | 0.15 psia |
| 1.5 psia |
|
| ||
| [1.034 kPa] |
| [10.34 kPa] |
|
| pressure | Figure 11 |
|
|
|
At a given pressure, the temperature at which a liquid will boil into a vapor is the same temperature at which the vapor will condense back into a liquid. This curve illustrates the pressures and corresponding temperatures at which water (the refrigerant) boils and condenses.
At atmospheric pressure (14.7 psia [101.3 kPa]), water boils and evaporates at 212 °F [100 °C]. When the pressure is decreased, water boils at a lower temperature. At the lower pressure, there is less force pushing against the water molecules, allowing them to separate easier.
Just like in the
9 |