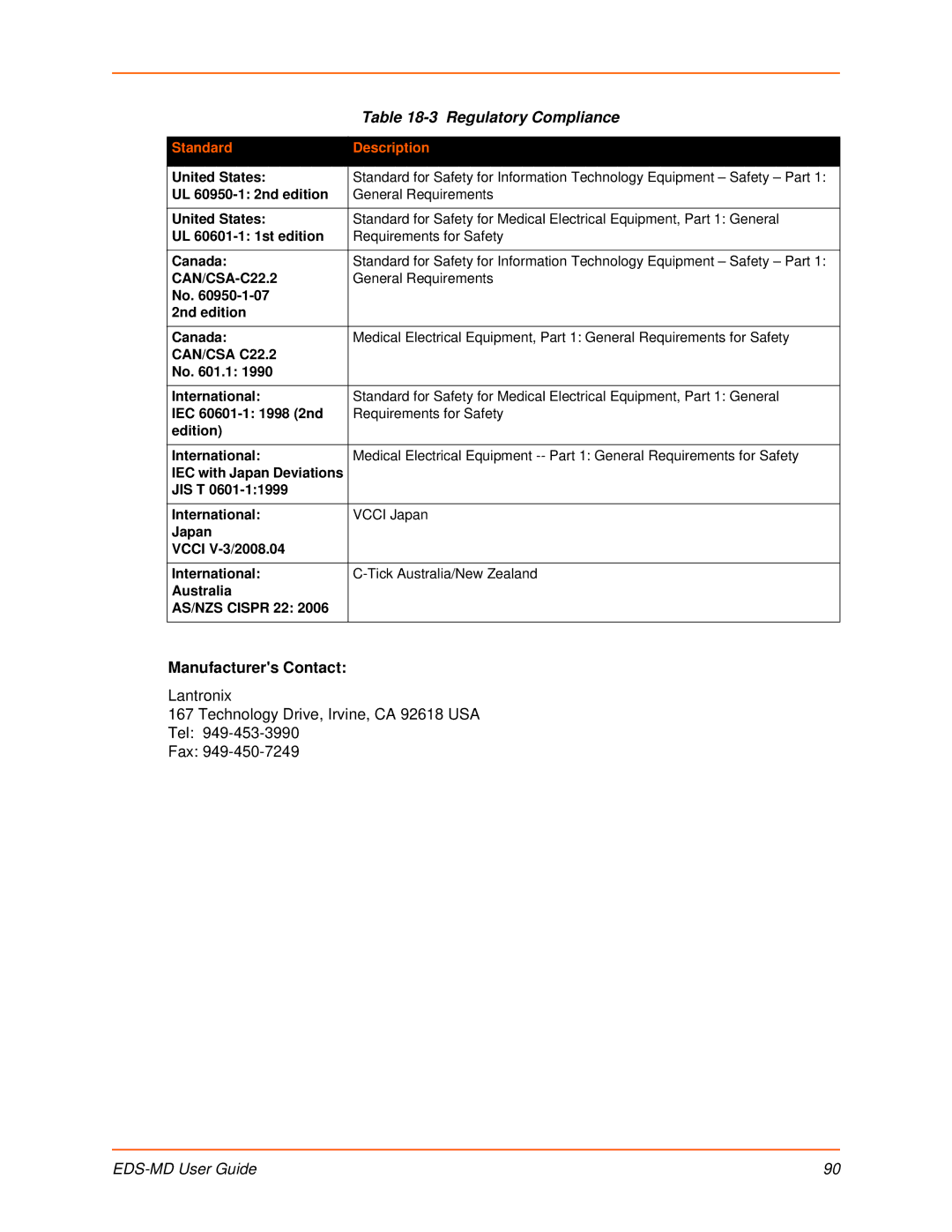
| Table |
|
|
Standard | Description |
|
|
United States: | Standard for Safety for Information Technology Equipment – Safety – Part 1: |
UL | General Requirements |
|
|
United States: | Standard for Safety for Medical Electrical Equipment, Part 1: General |
UL | Requirements for Safety |
|
|
Canada: | Standard for Safety for Information Technology Equipment – Safety – Part 1: |
General Requirements | |
No. |
|
2nd edition |
|
|
|
Canada: | Medical Electrical Equipment, Part 1: General Requirements for Safety |
CAN/CSA C22.2 |
|
No. 601.1: 1990 |
|
|
|
International: | Standard for Safety for Medical Electrical Equipment, Part 1: General |
IEC | Requirements for Safety |
edition) |
|
|
|
International: | Medical Electrical Equipment |
IEC with Japan Deviations |
|
JIS T |
|
|
|
International: | VCCI Japan |
Japan |
|
VCCI |
|
|
|
International: | |
Australia |
|
AS/NZS CISPR 22: 2006 |
|
|
|
Manufacturer's Contact:
Lantronix
167 Technology Drive, Irvine, CA 92618 USA
Tel:
Fax:
| 90 |