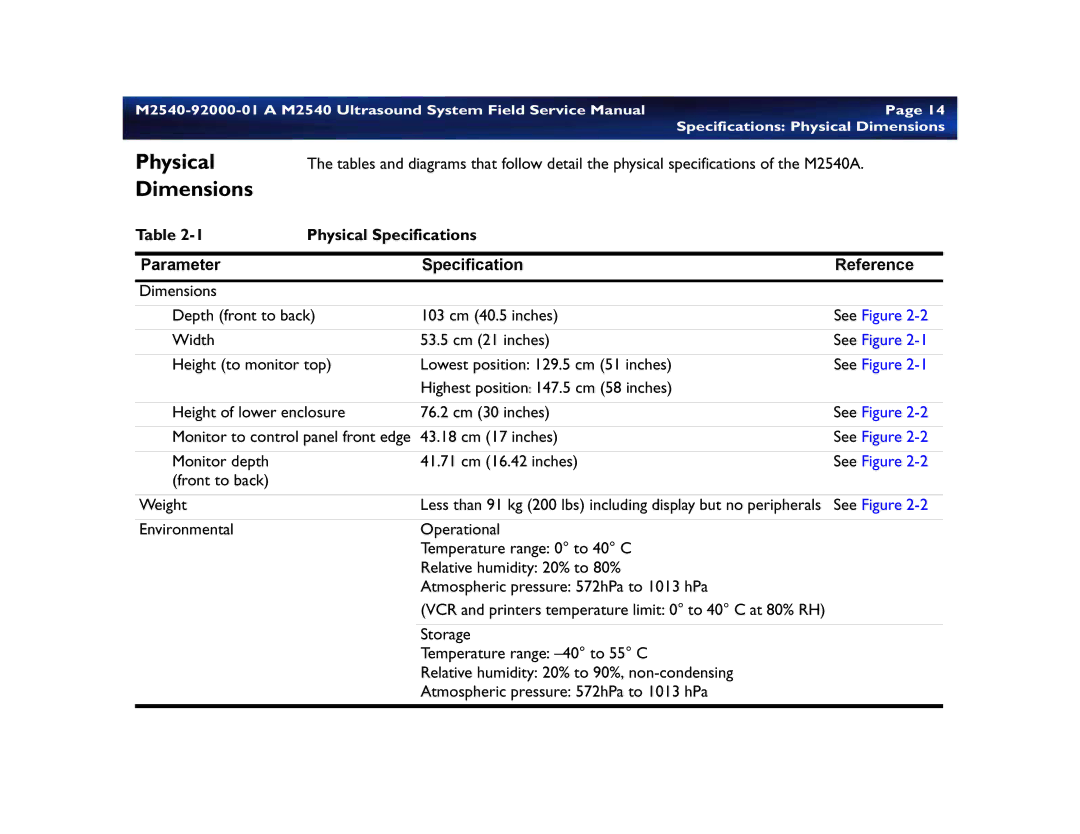
M2540 specifications
The Philips M2540 is a versatile and feature-rich patient monitor designed to enhance healthcare delivery in various clinical settings, from hospitals to outpatient facilities. With its advanced technology and user-friendly interface, the M2540 optimizes patient care through accurate monitoring and seamless data integration.One of the standout features of the Philips M2540 is its advanced parameter capabilities. It supports a wide range of measurements including ECG, heart rate, non-invasive blood pressure, temperature, and respiratory rate. This comprehensive monitoring allows healthcare professionals to obtain a holistic view of a patient's condition in real time, ensuring timely interventions when necessary.
In addition to the standard parameters, the M2540 offers optional modules for more specialized monitoring, such as multi-gas analysis and invasive blood pressure. This adaptability makes it suitable for various clinical environments, including surgical units, critical care, and ambulatory settings.
The M2540 is equipped with Philips' innovative IntelliVue technology, which enhances monitoring accuracy and reliability. IntelliVue incorporates advanced algorithms that reduce false alarms, thus easing the burden on healthcare staff and improving patient safety. Furthermore, the monitor features integrated patient data management capabilities, enabling seamless data transfer to electronic health records (EHRs) and facilitating better communication among the care team.
User experience is prioritized in the design of the M2540, featuring a clear and intuitive display that presents vital signs in a straightforward manner. The touchscreen interface is customizable, allowing users to arrange critical data according to their preferences. This ensures that clinicians can access vital information quickly and efficiently during high-pressure situations.
The portability of the M2540 is another significant advantage. It is designed to be lightweight and easily transportable, making it ideal for use in various locations, from operating rooms to patient transport. The long-lasting battery life ensures uninterrupted monitoring, even during transfers.
In summary, the Philips M2540 is a robust patient monitoring solution that combines advanced technology with user-centric design. Its comprehensive monitoring capabilities, IntelliVue technology, and portability make it an asset in any healthcare environment, ultimately enhancing the quality of patient care and outcomes.