MODEL 54eA | SECTION 11.0 |
| CALIBRATION - MONOCHLORAMINE |
SECTION 11.0
CALIBRATION - MONOCHLORAMINE
11.1 INTRODUCTION
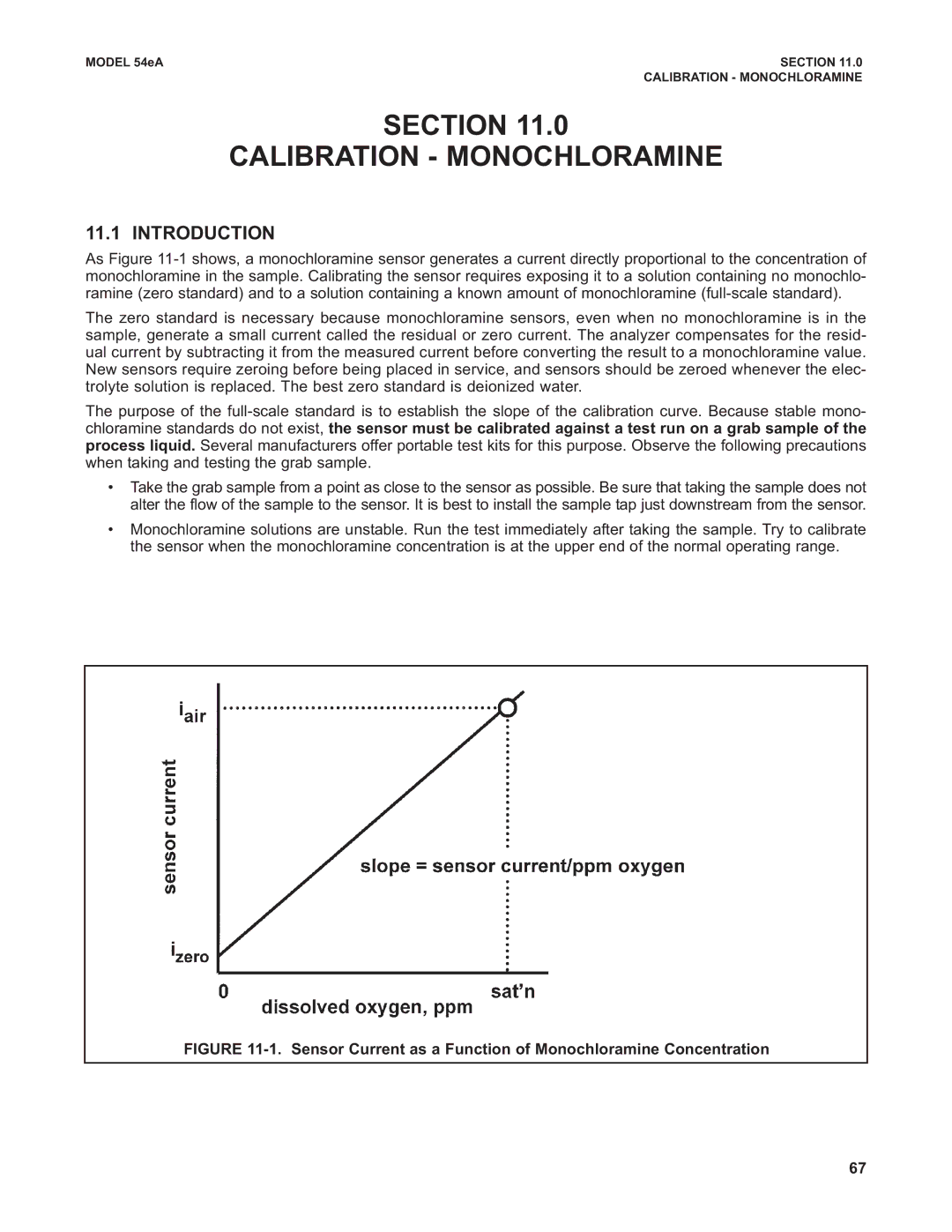
As Figure
The zero standard is necessary because monochloramine sensors, even when no monochloramine is in the sample, generate a small current called the residual or zero current. The analyzer compensates for the resid- ual current by subtracting it from the measured current before converting the result to a monochloramine value. New sensors require zeroing before being placed in service, and sensors should be zeroed whenever the elec- trolyte solution is replaced. The best zero standard is deionized water.
The purpose of the
•Take the grab sample from a point as close to the sensor as possible. Be sure that taking the sample does not alter the flow of the sample to the sensor. It is best to install the sample tap just downstream from the sensor.
•Monochloramine solutions are unstable. Run the test immediately after taking the sample. Try to calibrate the sensor when the monochloramine concentration is at the upper end of the normal operating range.
FIGURE 11-1. Sensor Current as a Function of Monochloramine Concentration
67