ADDENDUM TO MODEL 101E OPERATORS MANUAL
MODEL 102E TOTAL REDUCED SULFUR ANALYZER
MODEL 501 TRS THERMAL CONVERTER
with
Page
05514 Rev A1
TABLE OF CONTENTS
M102E/M501 TRS
Addendum to M101E Manual - P/N 04740 Rev A
10. TROUBLESHOOTING & REPAIR
LIST OF APPENDICES
8. INSTRUMENT MAINTENANCE
9. THEORY OF OPERATION
PREFACE
LIST OF FIGURES
LIST OF TABLES
Page
1. PREFACE
corner of the display any time the instrument is in SETUP mode
User Notes
1.1. Reference Numbering convention
2.1.1. M501-TRS Specifications
2. SPECIFICATIONS, APPROVALS AND WARRANTY
2.1. Specifications
2.2. EPA Equivalency Designation
2.3. CE Mark Compliance
3.2. Unpacking the M501-TRS
3. GETTING STARTED
3.1. Unpacking the M102E
Never disconnect electronic circuit boards, wiring harnesses or
the maximum operating temperature specification for the M102E 40C
3.2.1. M501-TRS Ventilation Clearance
that the rack/enclosure itself is adequately ventilated
Figure 3-2 M501-TRS Internal Layout
3.3. Internal Layouts
Figure 3-1 M102E Internal Layout
M102E INSTRUMENT CHASSIS
3.4. Internal Pneumatic Flow of the M102E & the M501-TRS
Table 3-1 TRS - SO2 Switching Valve Operating Modes
M501
Figure 3-4 M102E Rear Panel Layout
3.5. Rear Panel Layout for the M102E & M501-TRS
Alarm Output
5 Amp
3.6.1.1. M102E Analog Output Connections
3.6. Initial Setup
3.6.1. Electrical Connections
Figure 3-6 Analog Output Connector
M102E PNEMATIC CONNECTERS
3.6.1.2. M501-TRS Alarm Output Connections
3.6.2. Pneumatic Connections
M501-TRS PNEMATIC CONNECTERS
MODEL
102E
The exhaust from the instrument needs to be vented outside the
SPAN GAS
3.6.2.1. Connections with Internal Valve Options Installed
ZERO AIR
VENT if input is pressurized
Gas Dilution Calibrator
VENT
Option
Valve Option
3.7.2. Functional Check of the M102E
3.7. Initial Operation
3.7.1. Startup / Warm Up of the M102E
Possible Warning Messages at Start-Up
Process Variable
High Alarm LED Low Alarm LED
3.7.3. Startup / Warm Up of the M501-TRS
Display area
3.8. Initial Calibration
Page
4.2. Calibration Valves Options
4. OPTIONAL HARDWARE AND SOFTWARE
4.1. Rack Mount Kits Options 20a, 20b, 21, 22
OPTIONAL HARDWARE AND SOFTWARE
Zero/Span Valve Operating States
M102E INSTRUMENT CHASSIS
Table 4-2 IZS Valve Operating States
4.3.2. Addendum on CD Part number
4.3. Additional Manuals
4.3.1. Printed Manuals P/N
Figure 5-1 Analog Output Connector Key
5.1.1. M102E Analog Output Signals
5. M102E OPERATING INSTRUCTIONS
M102E OPERATING INSTRUCTIONS
5.2.1. M102E Analog I/O Configuration
5.2. SETUP - DIAG Using the Diagnostics Functions
5.1.2. Setting the M102E Gas Measurement Mode
Table 5-1 M102E gas Measurement Modes
5.3.1. M102E ID Code
5.3. SETUP - COMM Setting Up the M102E’s Communication Ports
5.2.2. M102E Test Channel Output
5.3.2. M102E Ethernet Host Name
CONTROL IN
5.4. Remote Operation of the Analyzer
5.4.1. Control Inputs
ZERO
5.4.2.1. M102E Hessen Protocol Gas ID List
5.4.2. Using the M102E with a Hessen Protocol Network
5 VDC Power Supply
SPAN
5.4.2.2. Setting Hessen Protocol Status Flags
Table 5-6 Default Hessen Status Bit Assignments
DO NOT OPERATE WITHOUT THE COVER OF THE M501TS CONVERTER INSTALLED
6. M501-TRS OPERATING INSTRUCTIONS
6.1. Basic M501-TRS Controls
Teledyne Instruments customer service
6.2. To Display The Current Temperature
Table 6-1 M501-TRS Temperature Controls and Definitions
6.3. To Manually Adjust the Converter Oven Temperature
DO NOT SET THE TEMPERATURE HIGHER THAN 1050OC
has reached a stable, constant temperature
6.4. Autotune the Temperature Controller
6.4.1. Initiating the Autotune Process
necessary to repeat the autotune procedure
6.5. M501TRS Alarm Relay Adjustment
6.4.2. Aborting the Autotune Process
Page
7.1. M102E Calibration
7. CALIBRATION PROCEDURES
USER NOTES
7.2. M501-TRS Calibration
Page
8. INSTRUMENT MAINTENANCE
INSTRUMENT MAINTENANCE
8.1.1. Maintaining the SO2 Scrubber
8.1. Additional and Updated Maintenance Procedures
8.1.1.1. Predicting When the SO2 Scrubber Should Be Replaced
8.1.1.2. Checking the Function of the SO2 Scrubber
8.1.1.3. Changing the SO2 Scrubber Material
Page
9.1.1. TRS Conversion
9. THEORY OF OPERATION
9.1. Measurement Principle
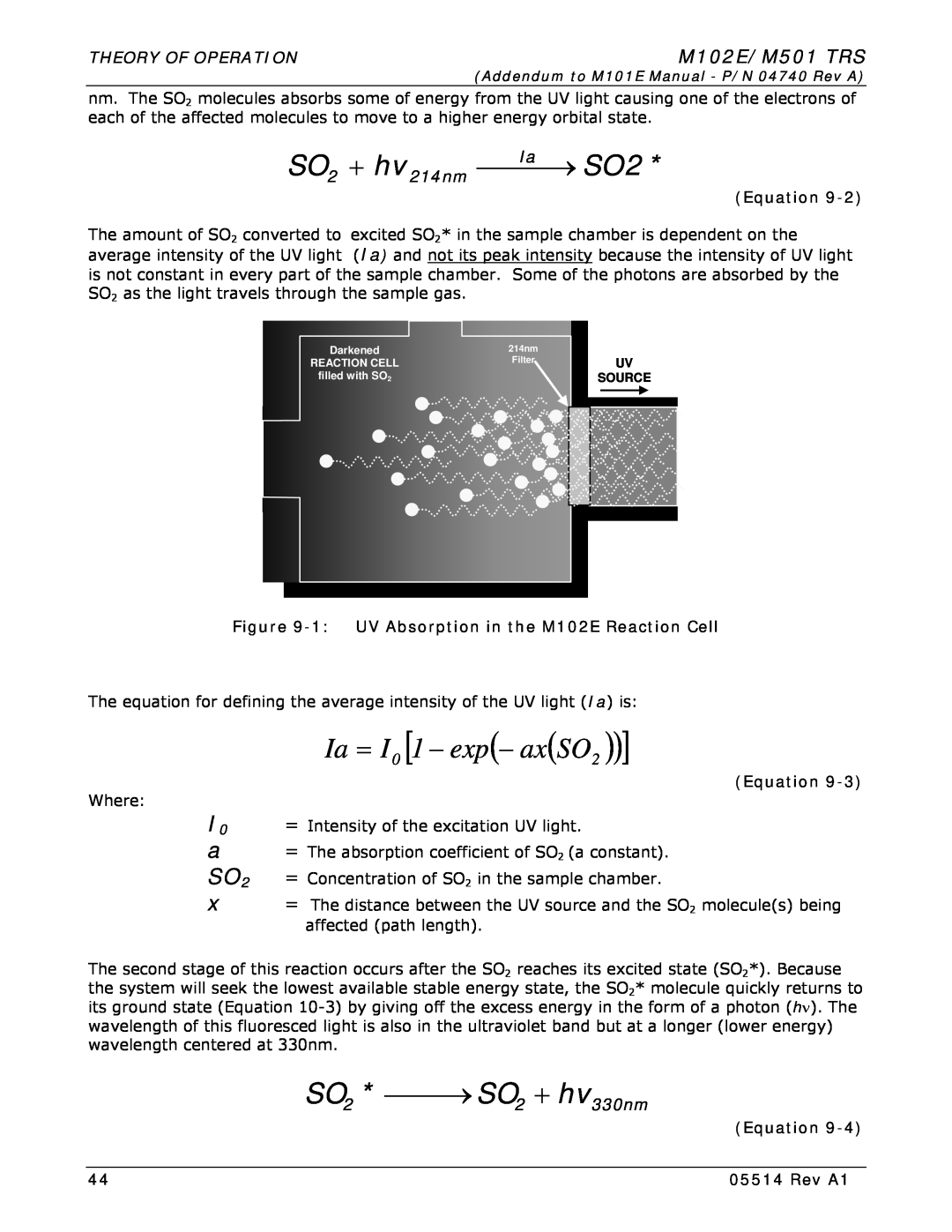
9.1.2. SO2 Ultraviolet Fluorescence
Ia = I0 1 − exp− axSO2
SO2 + hv214nm ⎯⎯Ia⎯→ SO2
SO2 * ⎯⎯→ SO2 + hv330nm
Figure 9-1 UV Absorption in the M102E Reaction Cell
Equation9-6
F = kSO2
kSO2 * ⎯⎯F⎯→ SO2 + hv330nm
9.2.1. UV Lamp Shutter & PMT Offset
9.2. The UV Light Path
9.3. Pneumatic Operation
9.3.1. Sample gas Flow
9.4.1. Sensor Module
PMT HOUSING
9.4. Electronic Operation
SAMPLE CHAMBERUV Lamp
Sample Air Housing Outlet O-Ring O-Ring Seal Seal
9.4.1.1. Sample Chamber
9.4.1.2. Sample Chamber Heating Circuit
Sample Air
HEATER
9.4.2.1. Thermal Switch
9.4.2. M501-TRS electronics
P-I-D CONTROLLER
9.4.2.2. Temperature Alarms and Alarm Output
10.1.1.2. M501-TRS Error Codes
10. TROUBLESHOOTING & REPAIR
10.1.1. Fault Diagnosis with Warning Messages
10.1.1.1. M102E Warning Messages
functioning
10.1.2. Fault Diagnosis with Test Functions
Table 10-2 Test Functions - Possible Causes for Out-Of-Range Values
10.2.1. TRS Converter Not Heating
10.2. M501-TRS Trouble shooting
10.3. Other Performance Problems
10.3.1. Excessive noise
10.4.2. Checking the Efficiency of the M501-TRS TRS Æ SO2 Converter
10.4. Subsystem Checkout
10.4.1. Checking the Efficiency of the M501-TRS SO2 Scrubber
10.5. Additional Repair Procedures
10.5.1. UV Lamp Adjustment and/or Replacement
Lamp Assembly
10.5.1.1. Adjusting the UV Lamp Peaking the Lamp
ALWAYS wear UV-Protective, Safety Glasses when working with the UV
Always grasp the main body of the lamp
10.5.1.2. Replacing the UV Lamp
Figure 10-1 Shutter Assembly - Exploded View
10.5.2. Replacing the UV filter/lens
Figure 10-2 Disassembling the Shutter Assembly
10.5.3. Replacing the PMT, HVPS or TEC
Figure 10-3 PMT Assembly - Exploded View
3. Remove the reaction cell assembly
10.5.4. M102E PMT Hardware Calibration FACTORY CAL
6. Locate the Preamp board see Figure
Figure 10-4 Pre-Amplifier Board Layout
Adjust the PMT until NORM PMT equals 1280 mV ± 10 mV
10.5.5. Replacing the TRS Converter Heating Tube
If a reporting range other than 500 ppb is used in this procedure
NORM PMT value of twice the ppb value of the span gas EXAMPLE
Be Careful
10.6. Manually Programming the M501-TRS Temperature Controller
The ceramic bobbins at each end of the heater assembly are fragile
element off
10.6.1. Temperature Controller Primary Menu Parameters
115V/60Hz with a set value of 1000C
Table 10-3 - Temperature Controller - Primary Parameter Settings
10.7. Technical Assistance
Table 10-4 - Temperature Controller - Primary Parameter Settings
M102E/M501 TRS
APPENDIX A-4 Model 102E Signal I/O Definitions
APPENDIX A - Version Specific Software Documentation
APPENDIX A-2 Model 102E Setup Variables Available Via Serial I/O
M102E/M501 TRS APPENDIX A - Version Specific Software Documentation
APPENDIX A-1 M102E Software Menu Trees, Revision A.2
SETUP
ENTER SETUP PASS
APPENDIX A-1 M102E Software Menu Trees, Revision A.2 SAMPLE
MSG 1,2
SAMPLE
TEST
CLR 1,3
TR2, 112, REPORTED
ACAL1
SO2, 111, REPORTED
DIAG
SAMPLE ENTER SETUP PASS
Figure A-4 Secondary Setup Menu DIAG
Table A-2
APPENDIX A-2 Setup Variables For Serial I/O, Revision A.2
Deleted Setup Variables for M102E Software Revision A.2
MEASUREMODE
Table A-4 Warning Messages deleted from M102 Software Revision A.2
setting of STABILFREQ and STABILSAMPLES
APPENDIX A-3 Warnings and Test Functions, Revision A.2
Table A-6 Test Functions Deleted from M102 Software Revision A.2
Rear board primary MUX analog inputs
APPENDIX A-4 M102E Signal I/O Definitions, Revision A.2
APPENDIX A-4 M102E Signal I/O Definitions, Revision A.2M102E/M501 TRS
CTEMPW
APPENDIX A-5 M102E iDAS Functions, Revision A.2
Trigger Event
Function
A-10
APPENDIX A-5 M102E iDAS Functions, Revision A.2
Table B-1 M102E Spare Parts List
APPENDIX B - M102E Spare Parts List
APPENDIX B - M102E Spare Parts List
M101E Manual - P/N 04740120 Rev A
05516 Rev A
TELEDYNE
Warranty/Repair Questionnaire Model 102E
M102E/M501 TRS
INSTRUMENTS
TELEDYNE
Table D-1 List of Included Electronic Schematics
APPENDIX D - ELECTRONIC SCHEMATICS
APPENDIX D - ELECTRONIC SCHEMATICS
Addendum to M101E Manual - P/N 04740 Rev A
M102E/M501 TRS
APPENDIX D - ELECTRONIC SCHEMATICS
User Notes
05518 Rev A