MODEL 3081 pH/ORP | SECTION 13.0 |
| pH MEASUREMENTS |
designed, the liquid junction potential is usually small and rel- atively constant. All three potentials depend on temperature. As discussed in Sections 13.5 and 13.6, the factor relating the cell voltage to pH is also a function of temperature.
The construction of each electrode and the electrical poten- tials associated with it are discussed in Sections 13.2, 13.3, and 13.4.
13.2 MEASURING ELECTRODE
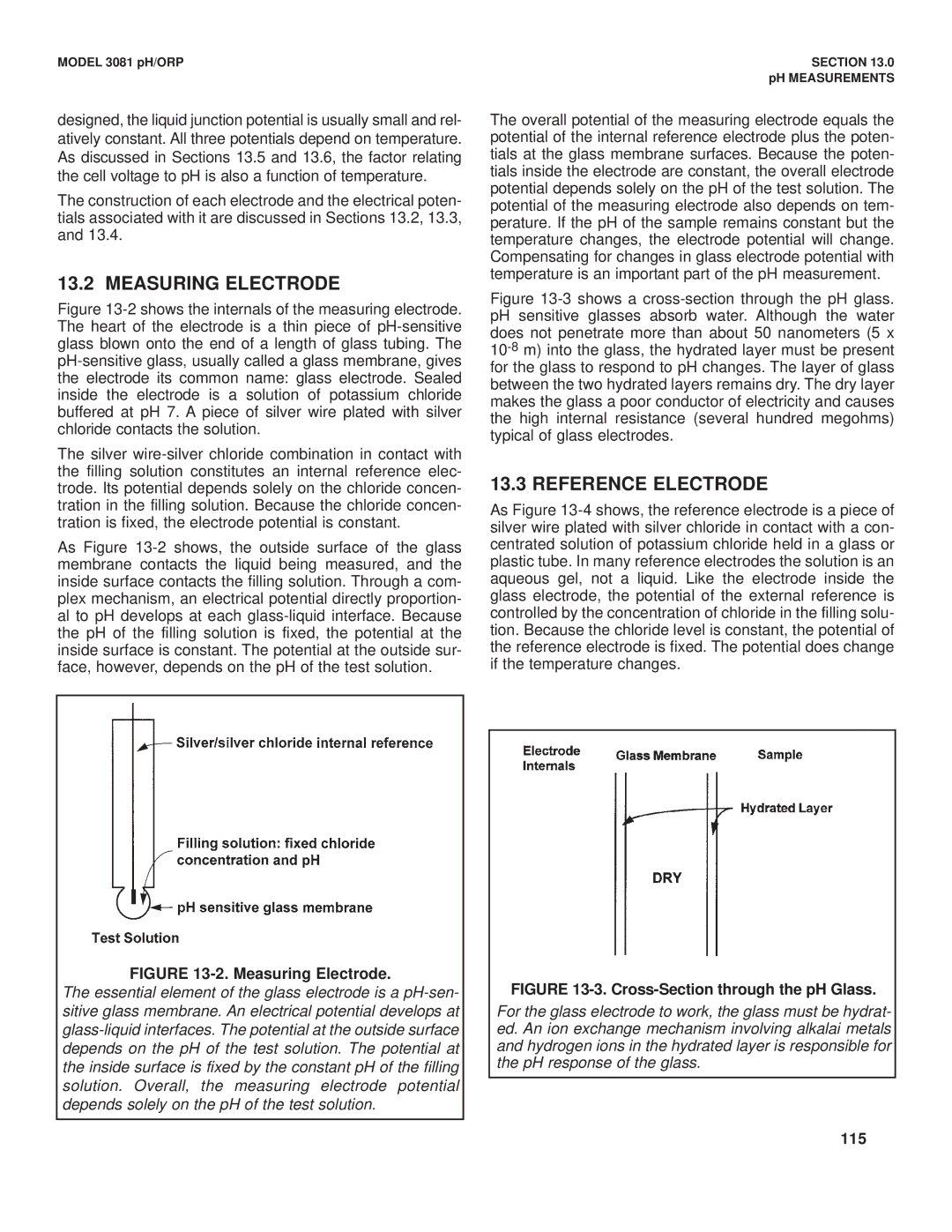
Figure 13-2 shows the internals of the measuring electrode. The heart of the electrode is a thin piece of pH-sensitive glass blown onto the end of a length of glass tubing. The pH-sensitive glass, usually called a glass membrane, gives the electrode its common name: glass electrode. Sealed inside the electrode is a solution of potassium chloride buffered at pH 7. A piece of silver wire plated with silver chloride contacts the solution.
The silver wire-silver chloride combination in contact with the filling solution constitutes an internal reference elec- trode. Its potential depends solely on the chloride concen- tration in the filling solution. Because the chloride concen- tration is fixed, the electrode potential is constant.
As Figure 13-2 shows, the outside surface of the glass membrane contacts the liquid being measured, and the inside surface contacts the filling solution. Through a com- plex mechanism, an electrical potential directly proportion- al to pH develops at each glass-liquid interface. Because the pH of the filling solution is fixed, the potential at the inside surface is constant. The potential at the outside sur- face, however, depends on the pH of the test solution.
FIGURE 13-2. Measuring Electrode.
The essential element of the glass electrode is a
depends on the pH of the test solution. The potential at the inside surface is fixed by the constant pH of the filling solution. Overall, the measuring electrode potential depends solely on the pH of the test solution.
The overall potential of the measuring electrode equals the potential of the internal reference electrode plus the poten- tials at the glass membrane surfaces. Because the poten- tials inside the electrode are constant, the overall electrode potential depends solely on the pH of the test solution. The potential of the measuring electrode also depends on tem- perature. If the pH of the sample remains constant but the temperature changes, the electrode potential will change. Compensating for changes in glass electrode potential with temperature is an important part of the pH measurement.
Figure 13-3 shows a cross-section through the pH glass. pH sensitive glasses absorb water. Although the water does not penetrate more than about 50 nanometers (5 x 10-8 m) into the glass, the hydrated layer must be present for the glass to respond to pH changes. The layer of glass between the two hydrated layers remains dry. The dry layer makes the glass a poor conductor of electricity and causes the high internal resistance (several hundred megohms) typical of glass electrodes.
13.3 REFERENCE ELECTRODE
As Figure
FIGURE 13-3. Cross-Section through the pH Glass.
For the glass electrode to work, the glass must be hydrat- ed. An ion exchange mechanism involving alkalai metals and hydrogen ions in the hydrated layer is responsible for the pH response of the glass.