MODEL 3081 pH/ORP | SECTION 11.0 |
| MAINTENANCE |
When using acid or alkaline solvents, be careful to keep the solvent away from the liquid junction. If the cleaning sol- vent contacts the junction, hydrogen ions (acid solvent) or hydroxide ions (alkaline solvent) will diffuse into the junc- tion. Because hydrogen and hydroxide ions have much greater mobility than other ions, they produce a large junction potential. When the electrode goes back in service, the hydrogen or hydroxide ions slowly diffuse out of the junction, causing the liquid junction potential and the pH reading to drift. It may take hours or days for the reading to stabilize. For a discussion of the influence of ion mobility on liquid junction potentials, see Section 13.4.
Consult the sensor instruction manual for additional information.
Always recalibrate the sensor after cleaning. If the sensor was cleaned with detergent or acid, soak the sensor in pH 4 or pH 7 buffer for at least an hour before calibrating.
11.3.3 Checking the Reference Electrode.
Some processes contain substances, for example, sulfides, that poison the reference electrode. Poisoning alters the electrode potential. For example, sulfide poisoning converts the reference electrode from a silver/silver chloride elec- trode into a silver/silver sulfide electrode, causing a shift in potential of several hundred millivolts.
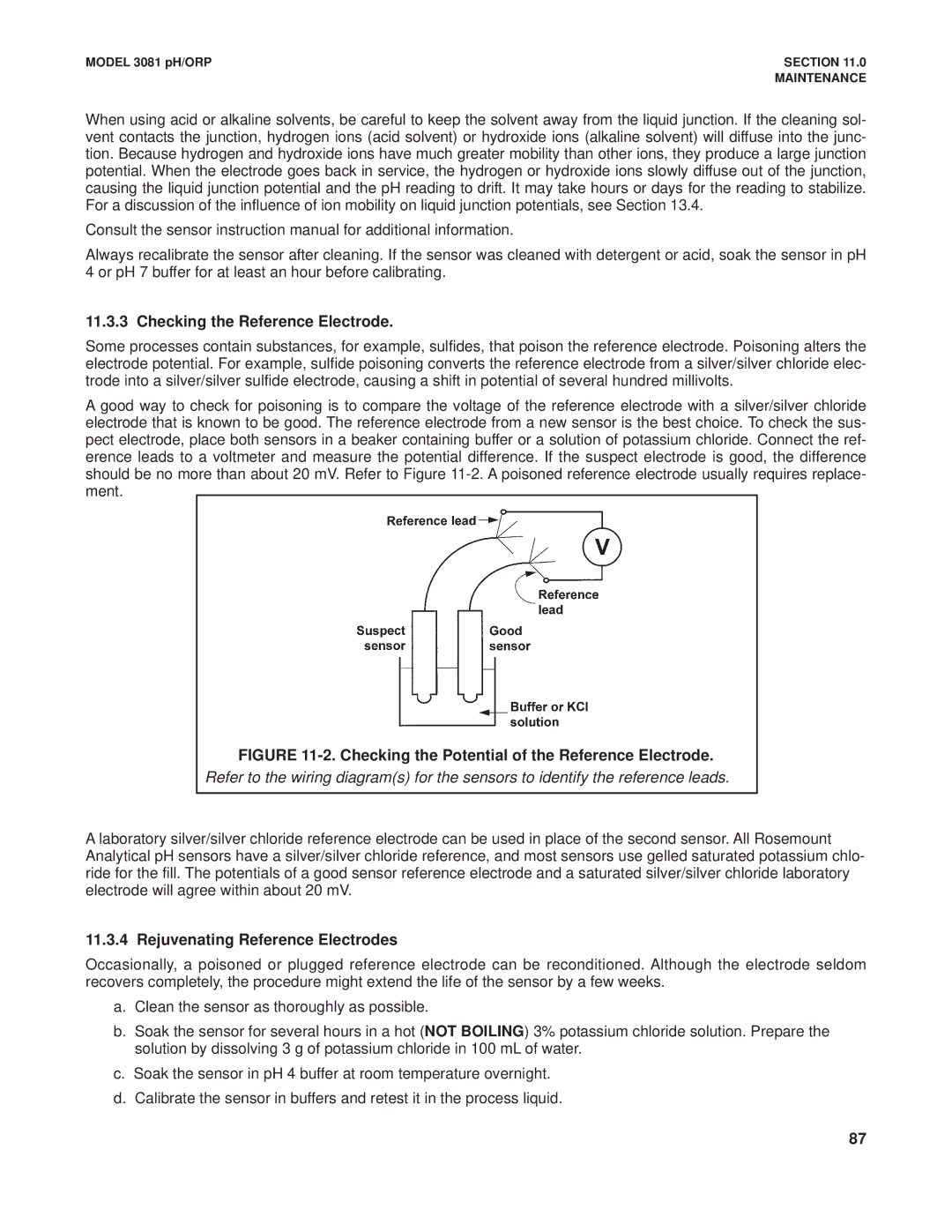
A good way to check for poisoning is to compare the voltage of the reference electrode with a silver/silver chloride electrode that is known to be good. The reference electrode from a new sensor is the best choice. To check the sus- pect electrode, place both sensors in a beaker containing buffer or a solution of potassium chloride. Connect the ref- erence leads to a voltmeter and measure the potential difference. If the suspect electrode is good, the difference should be no more than about 20 mV. Refer to Figure
FIGURE 11-2. Checking the Potential of the Reference Electrode.
Refer to the wiring diagram(s) for the sensors to identify the reference leads.
A laboratory silver/silver chloride reference electrode can be used in place of the second sensor. All Rosemount Analytical pH sensors have a silver/silver chloride reference, and most sensors use gelled saturated potassium chlo- ride for the fill. The potentials of a good sensor reference electrode and a saturated silver/silver chloride laboratory electrode will agree within about 20 mV.
11.3.4 Rejuvenating Reference Electrodes
Occasionally, a poisoned or plugged reference electrode can be reconditioned. Although the electrode seldom recovers completely, the procedure might extend the life of the sensor by a few weeks.
a.Clean the sensor as thoroughly as possible.
b.Soak the sensor for several hours in a hot (NOT BOILING) 3% potassium chloride solution. Prepare the solution by dissolving 3 g of potassium chloride in 100 mL of water.
c.Soak the sensor in pH 4 buffer at room temperature overnight.
d.Calibrate the sensor in buffers and retest it in the process liquid.
87