MODEL 3081 pH/ORP | SECTION 14.0 |
| ORP MEASUREMENTS |
is described by the following equation, called the Nernst equation:
| 0.1987 (t + 273.15) | log | [Fe+2] |
| |||
E = E° | - |
|
|
| (2) | ||
n | [Fe+3] | ||||||
|
|
|
| ||||
In the Nernst equation, E is the electrode potential and E° is the standard electrode potential, both in millivolts, t is temperature in °C, n is the number of electrons transferred (n = 1 in the present case), and [Fe+2] and [Fe+3] are the concentrations of iron (II) and iron (III) respectively. There are several ways of defining the standard electrode potential, E°. No matter which defi- nition is used, the standard electrode potential is sim- ply the electrode potential when the concentrations of iron (II) and iron (III) have defined standard values.
Equation 2 shows that the electrode potential is con- trolled by the logarithm of the ratio of the concentration of iron (II) to iron (III). Therefore, at 25°C if the ratio changes by a factor of ten, the electrode potential changes by
0.1987 (25 + 273.15)
-log 10 = - 59.2 mV 1
As the expression above shows, the voltage change is also directly proportional to temperature and inversely proportional to the number of electrons transferred.
14.7 INTERPRETING ORP MEASUREMENTS
Interpreting ORP and changes in ORP requires great caution. There are several concepts to keep in mind concerning industrial ORP measurements.
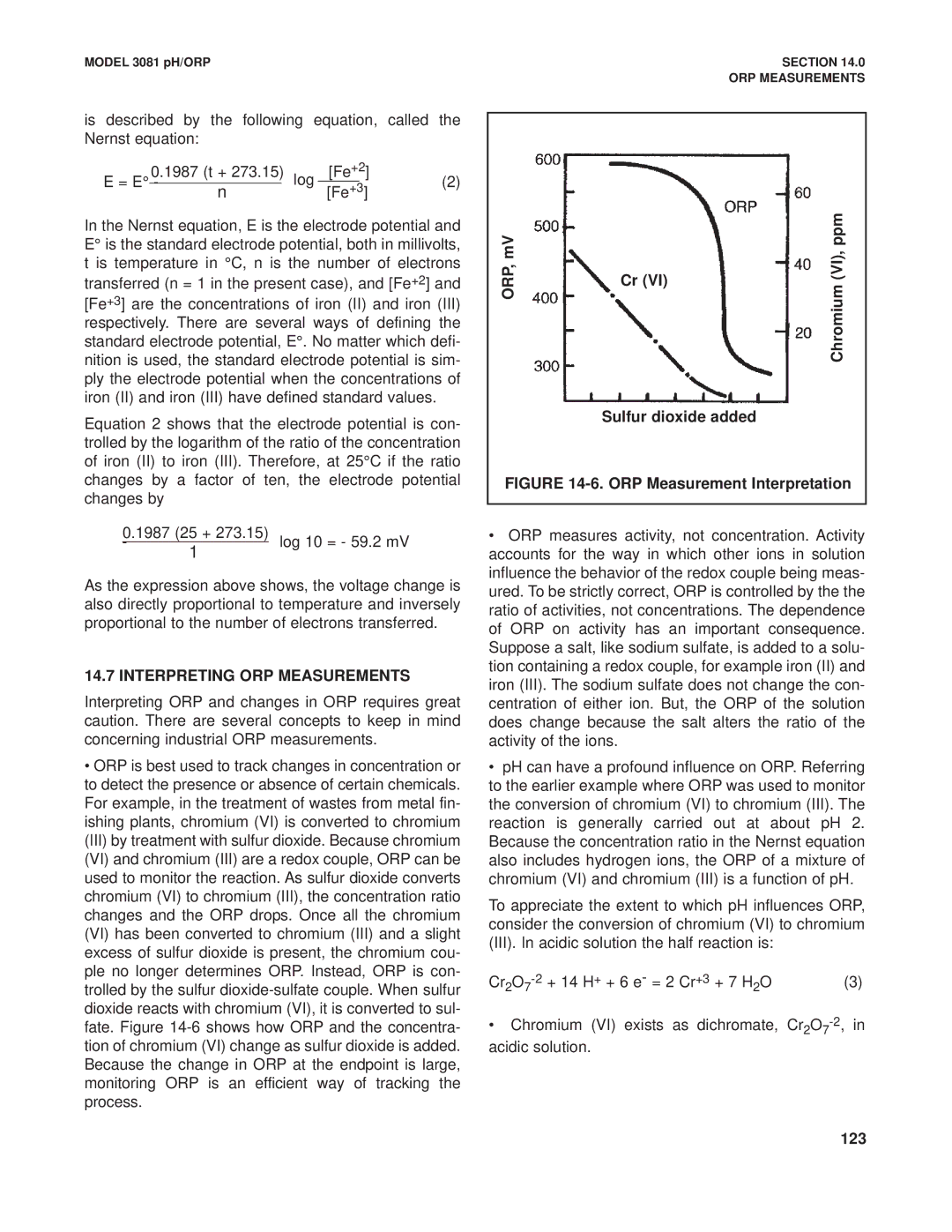
•ORP is best used to track changes in concentration or to detect the presence or absence of certain chemicals. For example, in the treatment of wastes from metal fin- ishing plants, chromium (VI) is converted to chromium (III) by treatment with sulfur dioxide. Because chromium (VI) and chromium (III) are a redox couple, ORP can be used to monitor the reaction. As sulfur dioxide converts chromium (VI) to chromium (III), the concentration ratio changes and the ORP drops. Once all the chromium (VI) has been converted to chromium (III) and a slight excess of sulfur dioxide is present, the chromium cou- ple no longer determines ORP. Instead, ORP is con- trolled by the sulfur
ORP,mV |
|
|
| (VI),Chromiumppm |
|
| Cr (VI) |
| |
|
|
|
|
|
| Sulfur dioxide added | |||
FIGURE 14-6. ORP Measurement Interpretation
•ORP measures activity, not concentration. Activity accounts for the way in which other ions in solution influence the behavior of the redox couple being meas- ured. To be strictly correct, ORP is controlled by the the ratio of activities, not concentrations. The dependence of ORP on activity has an important consequence. Suppose a salt, like sodium sulfate, is added to a solu- tion containing a redox couple, for example iron (II) and iron (III). The sodium sulfate does not change the con- centration of either ion. But, the ORP of the solution does change because the salt alters the ratio of the activity of the ions.
•pH can have a profound influence on ORP. Referring to the earlier example where ORP was used to monitor the conversion of chromium (VI) to chromium (III). The reaction is generally carried out at about pH 2. Because the concentration ratio in the Nernst equation also includes hydrogen ions, the ORP of a mixture of chromium (VI) and chromium (III) is a function of pH.
To appreciate the extent to which pH influences ORP, consider the conversion of chromium (VI) to chromium (III). In acidic solution the half reaction is:
(3) |
•Chromium (VI) exists as dichromate,
acidic solution.