MODEL 3081 pH/ORP
14.2 MEASURING ELECTRODE
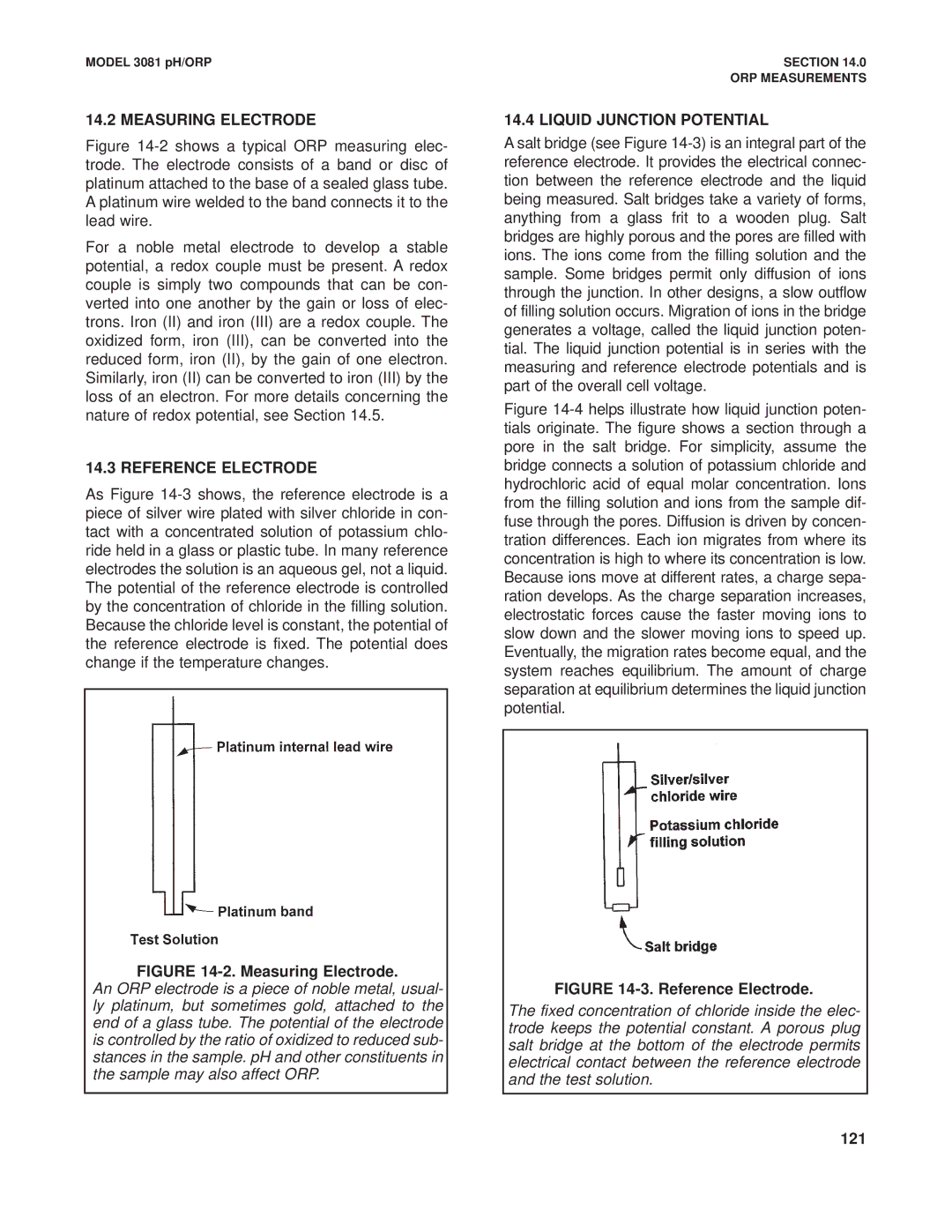
Figure 14-2 shows a typical ORP measuring elec- trode. The electrode consists of a band or disc of platinum attached to the base of a sealed glass tube. A platinum wire welded to the band connects it to the lead wire.
For a noble metal electrode to develop a stable potential, a redox couple must be present. A redox couple is simply two compounds that can be con- verted into one another by the gain or loss of elec- trons. Iron (II) and iron (III) are a redox couple. The oxidized form, iron (III), can be converted into the reduced form, iron (II), by the gain of one electron. Similarly, iron (II) can be converted to iron (III) by the loss of an electron. For more details concerning the nature of redox potential, see Section 14.5.
14.3 REFERENCE ELECTRODE
As Figure
FIGURE 14-2. Measuring Electrode.
An ORP electrode is a piece of noble metal, usual- ly platinum, but sometimes gold, attached to the end of a glass tube. The potential of the electrode is controlled by the ratio of oxidized to reduced sub- stances in the sample. pH and other constituents in the sample may also affect ORP.
SECTION 14.0
ORP MEASUREMENTS
14.4 LIQUID JUNCTION POTENTIAL
A salt bridge (see Figure
Figure 14-4 helps illustrate how liquid junction poten- tials originate. The figure shows a section through a pore in the salt bridge. For simplicity, assume the bridge connects a solution of potassium chloride and hydrochloric acid of equal molar concentration. Ions from the filling solution and ions from the sample dif- fuse through the pores. Diffusion is driven by concen- tration differences. Each ion migrates from where its concentration is high to where its concentration is low. Because ions move at different rates, a charge sepa- ration develops. As the charge separation increases, electrostatic forces cause the faster moving ions to slow down and the slower moving ions to speed up. Eventually, the migration rates become equal, and the system reaches equilibrium. The amount of charge separation at equilibrium determines the liquid junction potential.
FIGURE 14-3. Reference Electrode.
The fixed concentration of chloride inside the elec- trode keeps the potential constant. A porous plug salt bridge at the bottom of the electrode permits electrical contact between the reference electrode and the test solution.