MODEL 3081 pH/ORP | SECTION 13.0 |
| pH MEASUREMENTS |
4. the liquid junction potential.
The second term, 0.1984 T pH, is the potential (in mV) at the outside surface of the pH glass. This potential depends on temperature and on the pH of the sample. Assuming temperature remains constant, any change in cell voltage is caused solely by a change in the pH of the sample. Therefore, the cell voltage is a measure of the sample pH.
Note that a graph of equation 1, E(T) plotted against pH, is a straight line having a
13.6 GLASS ELECTRODE SLOPE
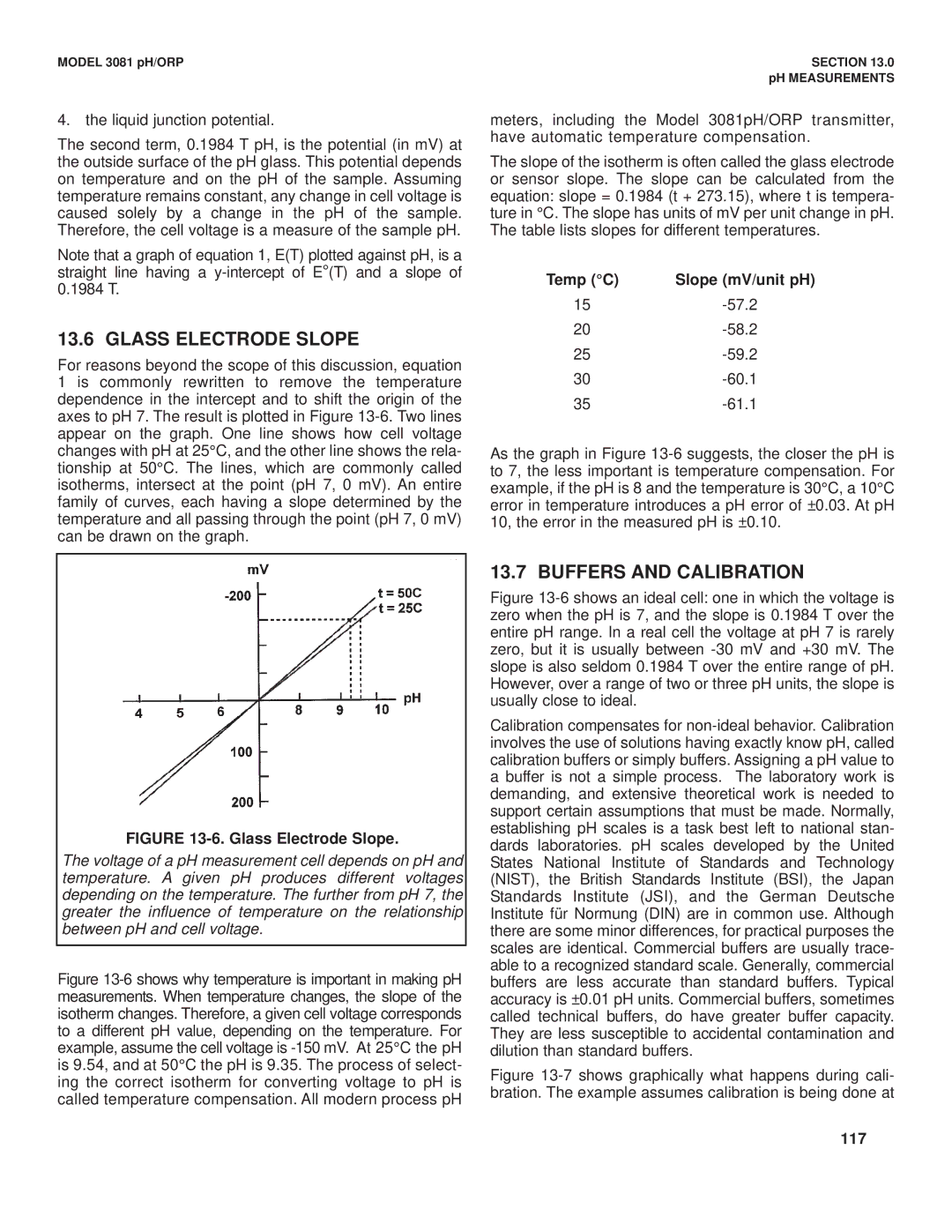
For reasons beyond the scope of this discussion, equation 1 is commonly rewritten to remove the temperature dependence in the intercept and to shift the origin of the axes to pH 7. The result is plotted in Figure
meters, including the Model 3081pH/ORP transmitter, have automatic temperature compensation.
The slope of the isotherm is often called the glass electrode or sensor slope. The slope can be calculated from the equation: slope = 0.1984 (t + 273.15), where t is tempera- ture in °C. The slope has units of mV per unit change in pH. The table lists slopes for different temperatures.
Temp (°C) | Slope (mV/unit pH) |
15 | |
20 | |
25 | |
30 | |
35 |
As the graph in Figure
FIGURE 13-6. Glass Electrode Slope.
The voltage of a pH measurement cell depends on pH and temperature. A given pH produces different voltages depending on the temperature. The further from pH 7, the greater the influence of temperature on the relationship between pH and cell voltage.