MODEL 3081 pH/ORP | SECTION 13.0 |
| pH MEASUREMENTS |
13.4 LIQUID JUNCTION POTENTIAL
The salt bridge (see Figure
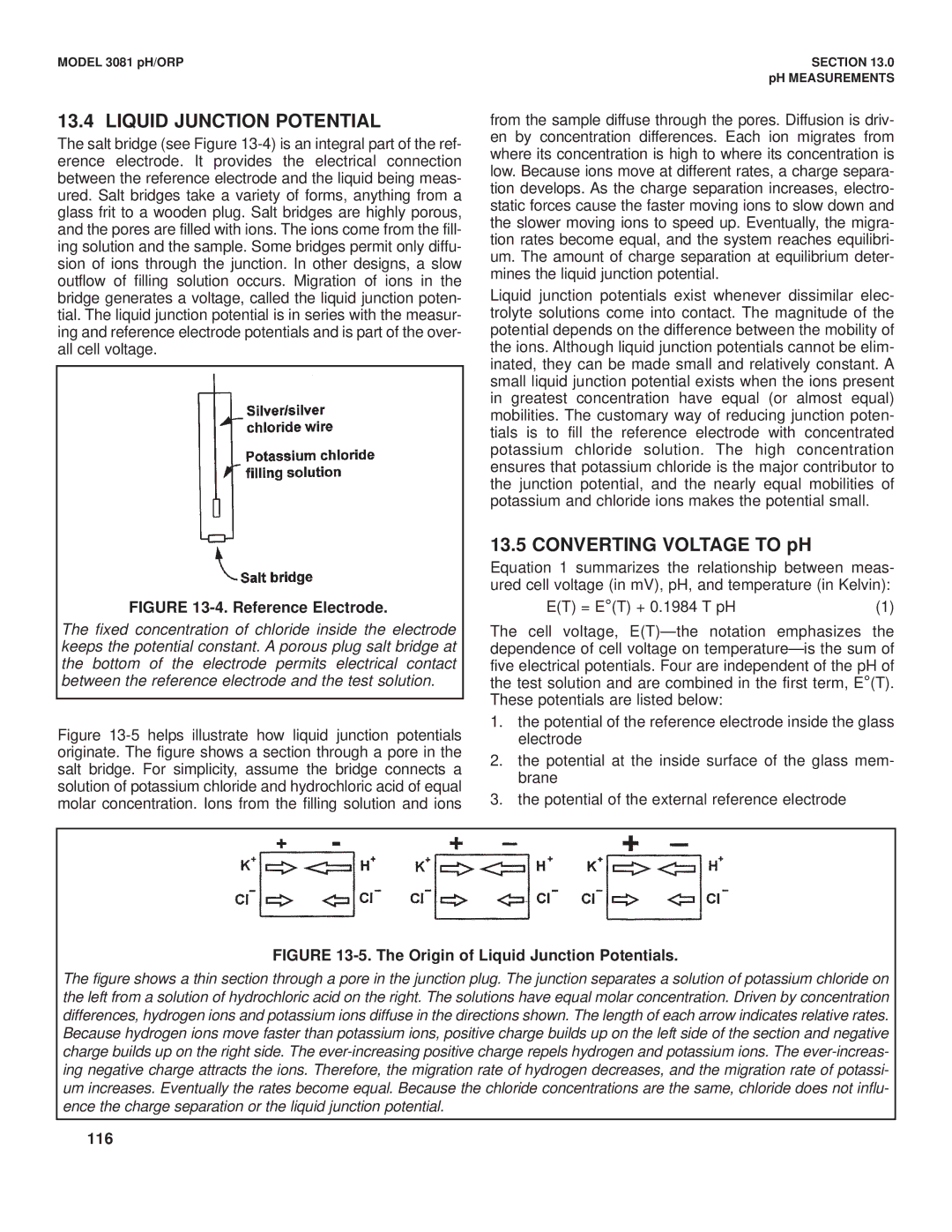
FIGURE 13-4. Reference Electrode.
The fixed concentration of chloride inside the electrode keeps the potential constant. A porous plug salt bridge at the bottom of the electrode permits electrical contact between the reference electrode and the test solution.
Figure 13-5 helps illustrate how liquid junction potentials originate. The figure shows a section through a pore in the salt bridge. For simplicity, assume the bridge connects a solution of potassium chloride and hydrochloric acid of equal molar concentration. Ions from the filling solution and ions
from the sample diffuse through the pores. Diffusion is driv- en by concentration differences. Each ion migrates from where its concentration is high to where its concentration is low. Because ions move at different rates, a charge separa- tion develops. As the charge separation increases, electro- static forces cause the faster moving ions to slow down and the slower moving ions to speed up. Eventually, the migra- tion rates become equal, and the system reaches equilibri- um. The amount of charge separation at equilibrium deter- mines the liquid junction potential.
Liquid junction potentials exist whenever dissimilar elec- trolyte solutions come into contact. The magnitude of the potential depends on the difference between the mobility of the ions. Although liquid junction potentials cannot be elim- inated, they can be made small and relatively constant. A small liquid junction potential exists when the ions present in greatest concentration have equal (or almost equal) mobilities. The customary way of reducing junction poten- tials is to fill the reference electrode with concentrated potassium chloride solution. The high concentration ensures that potassium chloride is the major contributor to the junction potential, and the nearly equal mobilities of potassium and chloride ions makes the potential small.
13.5 CONVERTING VOLTAGE TO pH
Equation 1 summarizes the relationship between meas- ured cell voltage (in mV), pH, and temperature (in Kelvin):
E(T) = E°(T) + 0.1984 T pH | (1) |
The cell voltage,
1.the potential of the reference electrode inside the glass electrode
2.the potential at the inside surface of the glass mem- brane
3.the potential of the external reference electrode