HP 50g / 49g+ / 48gII graphing calculator
Advanced user’s reference manual
Page
Acknowledgements
Printing History
Page
Contents
Names
Algb
Alog
Amort
317
Delalarm
Delay
→COL
COL→
359
Findalarm
EXP2POW
Expan
Expand
Halt
IBP
IDIV2
Iferr
List
MAX
Maxr
MIN
Not
PA2B2
Parametric
Parity
Parsurface
3183
RND
Rnrm
Roll
Rolld
3226
Tabvar
→TAG
Tail
TAN
Wireframe
Updir
Utpc
Utpf
Contents
514
→ALG Apeek ARM→ ASM ASM→
610
Alrmdat
CST Exited Expr Iopar MASD.INI
Toff Tpar
Vpar
Contents of a Program
Understanding Programming
Program Results
Calculations in a Program
Examples of Program Actions
To enter commands and other objects in a program
Entering and Executing Programs
To enter a program
To store or name a program
To stop an executing program
3Q!Ì*4`3/*@Ï
3Q!Ì*4#3/* @Ë@Ï
@% @Ér # O4 /3 *!Ì Q3 @Ë@Ï
Osph K
Level
Program Keys Comments
To switch between entry modes
Viewing and Editing Programs
To view or edit a program
@%SPH% ˜
Using Local Variables
Creating Programs on a Computer
Creating Local Variables
`J!%SPH% @%SPH% ˜
Program Comments
`OSPHLV K
Evaluating Local Names
Defining the Scope of Local Variables
Compiled Local Variables
Creating UserDefined Functions as Programs
Using Tests and Conditional Structures
Testing Conditions
Key Programmable Description Command
To include a test in a program
Using Logical Functions
Keys Programmable Description Command
Logical Functions
TEST% L
Using Conditional Structures and Commands
To enter if then END in a program
Press !%BRCH% !%IF%
To enter IFT in a program
To enter if then Else END in a program
Press !%BRCH% @%#IF#%
To enter Ifte in a program or in an algebraic
Press !%BRCH% L!%IFTE%
To enter Case END in a program
Conditional Examples
Otst K
26 `52 J %TST%
While … Repeat … END
Using Loop Structures
Using Definite Loop Structures
Start Next Structure
« … start finish Start loopclause Next … »
To enter Start Next in a program
Press !%BRCH% !%START%
Start Step Structure
« … start finish Start loopclause increment Step … »
To enter Start Step in a program
Press ! %BRCH% … %START%
For Next Structure
« … start finish for counter loopclause Next … »
To enter for Next in a program
Press !%BRCH% ! %FOR%
For Step Structure
« … start finish for counter loopclause increment Step … »
To enter for Step in a program
Press ! %BRCH% … %FOR%
Using Indefinite Loop Structures
Do Until END Structure
« … do loopclause Until testclause END … »
To enter do Until END in a program
Duplicates n, stores the value into n
While Repeat END Structure
« … While testclause Repeat loopclause END … »
To enter While Repeat END in a program
Press !%BRCH% ! %WHILE%
Press ! #MEM# %ARITH% %INCR% or %DECR%
Using Loop Counters
To enter Incr or Decr in a program
Using Summations Instead of Loops
∑ j
Using Flags
Setting, Clearing, and Testing Flags
Types of Flags
To set, clear, or test a flag
Recalling and Storing the Flag States
Example
Month/day/year format
Day.month.year format
Using Subroutines
To recall the current flag states
Execute Rclf !L %MODES% %FLAG% L%RCLF%
To change the current flag states
` 8 J %TORSV%
Torsa K
Torsv K
To singlestep from the start of a program
To turn off the Halt annunciator at any time
Press !LL %RUN% %KILL%
@·J6 `8 `O%TORSV% !LL%RUN% %DBUG%
@·J10 `12 O%TORSV% !LL%RUN% %DBUG%
To singlestep when the next step is a subroutine
To singlestep from the middle of a program
Trapping Errors
Causing and Analyzing Errors
To cause a userdefined error to occur in a program
SingleStep Operations
To artificially cause a builtin error to occur in a program
To analyze an error in a program
Error Trapping Commands
%ERROR%
Making an Error Trap
« … Iferr trapclause then errorclause END … »
Press !LL %ERROR% !%IFERR%
Iferr then END Structure
To enter Iferr then Else END in a program
Press !LL %ERROR% @%IFERR%
Iferr then Else END Structure
Data Input Commands
Using PROMPT, Cont for Input
Key Command Description
Input
`OTPROMPT ‰
@·J %TPROM%
To respond to Halt while running a program
Using Disp Freeze HALT, Cont for Input
To enter Disp Freeze Halt in a program
Using INPUT, Enter for Input
« … promptstring commandline Input OBJ→ … »
To enter Input in a program
To respond to Input while running a program
%VSPH%
Commandline cursorposition operatingoptions
To design the commandline string for Input
To include Input options
Cursorposition
`O Tinput ‰
To process the result string from Input
Press @ Õ ! Ê a @
%TINPU%
Using Inform and Choose for Input
To set up an input form
To set up a choose box
`OPHONES ‰
Using Wait for Keystroke Input
Beeping to Get Attention
To enter Beep in a program
To enter Wait in a program
Using KEY for Keystroke Input
Data Output Commands
Output
OUT%
Labeling Output with Tags
Labeling and Displaying Output as Strings
To label a result with a tag
%TTAG% 1.5 ˜1.85 `
Pausing to Display Output
Using Msgbox to Display Output
To set up a message box
`OTSTRING ‰
Using Menus with Programs
Using Menus for Input
Using Menus to Run Programs
To create a menubased application
Olst ‰
SI%
@·J%EIZ%
Turning Off the Calculator from a Program
%% !Ü.37~@6 68 %%I%% !%%Z%%
@%%I%% 2*%%I%% !%%E%%
To turn off the calculator in a program
Page
RPL Programming Examples
Fibonacci Numbers
FIB1 Fibonacci Numbers, Recursive Version
FIB1% !Ü10 N
FIB2 Fibonacci Numbers, Loop Version
%FIB1%
10 %FIB2%
FIB2 program listing
%FIB2%
Fibt Comparing ProgramExecution Time
Techniques used in Fibt
Structured programming. Fibt calls both FIB1 and FIB2
Required Programs
Displaying a Binary Integer
PAD Pad with Leading Spaces
13 %FIBT%
Techniques used in PAD
Preserve Save and Restore Previous Status
PAD program listing
`OPAD K
Techniques used in Preserve
`OPRESERVE K
Bdisp Binary Display
Preserve program listing
Techniques used in Bdisp
PAD
Bdisp program listing
`OBDISP K
144 %BDISP%
Median of Statistics Data
Tile Percentile of a list
Median Median of Statistics Data
Techniques used in %TILE
Tile program listing
`O%TILE K
Techniques used in Median
Required Program
Expanding and Collecting Completely
`OMEDIAN K
18 `12 `˜šš
11 `1 `
Multi Multiple Execution
Techniques used in Multi
Multi program listing
`OMULTI K
Exco Expand and Collect Completely
Techniques used in Exco
Exco program listing
`OEXCO K
Minimum and Maximum Array Elements
MNX Minimum or Maximum Element-Version
Ü4 *Y+Z +
User and system flags for logic control
MNX program listing
`OMNX K
Techniques used in MNX2
MNX2 Minimum or Maximum Element-Version
12 `56 `˜šš 45 `1 `
MNX2 program listing
Applying a Program to an Array
%MNX2%
Techniques used in Aply
Aply program listing
Make sure the flag 1 is clear to begin
Converting Between Number Bases
`OAPLY K
Ô3†2W†4` ‚Å 3 QA *7 `J %APLY% H#DISP ˜˜
Techniques used in nBASE
NBASE program listing
Verifying Program Arguments
1000 `23 J %NBASE%
Names Check List for Exactly Two Names
Techniques used in Names
Names program listing
`ONAMES K
VFY program listing
VFY Verify Program Argument
Techniques used in VFY
Converting Procedures from Algebraic to RPN
Oben `
Ojeff ` Osarah `
%LIST% %²LIST%
Techniques used in →RPN
→RPN program listing
Techniques used in BER
Bessel Functions
XQ3 `%²RPN%
BER program listing
`OBER K
%BER%
%BER% !Ü2
Animation of Successive Taylor’s Polynomials
Sintp Converting a Plot to a Graphics Object
Techniques used in Sintp
Sintp program listing
Setts Superimposing Taylor’s polynomials
Techniques used in Setts
Setts program listing
`OSETTS K
TSA Animating Taylor’s Polynomials
Techniques used in TSA
TSA program listing
`OTSA K
Programmatic use of matrices and statistics commands
Programmatic Use of Statistics and Plotting
Techniques used in PIE
Temporary menu for data input
Angle
`OPIE K
983 %SLICE% 416 %SLICE% 85 %SLICE%
Trace Mode
Techniques used in αENTER and ßENTER
ΑENTER program listing
ßENTER program listing
InverseFunction Solver
Techniques used in Rootr
Rootr program listing
`OROOTR K
Animating a Graphical Image
@Å@É x †O3.7
%X²FX% ` 599.5 ` 1 %ROOTR%
Techniques used in Walk
`OWALK K
Introduction
Acos
Type
Input/Output
Parallel Processing with Lists
How Commands Are Alphabetized
Other Provided Details
Computer Algebra System Commands and Functions
Classification of Operations
Backup identifier
Realnumber time or angle in hoursminutessecond s format
Library identifier
Binary integer
ACK
Abcuv
ABS
Ackall
Type Description
ACOS2S
ASIN, ATAN, COS, Isol
Acosh
ASIN2C, ASIN2T, ATAN2S
Acosh z
Symb ACOSHsymb
ADD
Addtmod
Addtoreal
Algb
Alog
10z
TVM, TVMBEG, TVMEND, Tvmroot
Amort
Principal Interest Balance
Animate
ANS
ARC
Access …µAPPLY Input/Output
Apply
Archive
ARG
Access …µARRY→ Input/Output
Arit
ARRY→
→ARRY
Access !¼ ¼is the leftshift of the Skey
Asinh
ASIN2C
ASIN2T
ASN
Asinh z
Symb ASINHsymb
ACOSH, ATANH, ISOL, Sinh
Access … ÃL BIT ASR
ASR
Assume
Skey
Atan
ADDTOREAL, Unassume
ATAN2S
Atan z
Symb ATANsymb
ACOS, ASIN, ISOL, TAN
Access …µATICK
Atanh
Atick
Atanh z
Access …µATTACH Input/Output
Attach
Augment
#n #m
Access …µAUTO Input/Output None
Auto
Axes
BAR
Access …µAXES Input/Output
AXL
AXM
Atick xaxis label yaxis label
AXQ
BAR
AXL, AXQ
AXL, AXM, GAUSS, QXA
Access …µBAR Input/Output None
Barplot
Basis
Baud
Access …µBESTFIT Input/Output None
Access … Ã BIN
Beep
Bestfit
Access !L Pict BOX Is the leftshift of the Nkey
Bins
Blank
BOX
Access …µBUFLEN Input/Output
Buflen
Bytes
#n1 #m1 #n2 #m2 X1, y1 X2, y2
Cascfg
Access … Ã B → R
C2P
Help
Cascmd
Case
Ceil
Centr
Xunit Nunit Symb CEILsymb
FLOOR, IP, RND, Trnc
Chinrem
Cholesky
CHR
Command Result
Choose
Circ
Cksm
NUM, POS
REPL, SIZE, SUB
Clear
Clkadj
Cllcd
Closeio
CLΣ
Clusr
Clvar
Cmplx
COL
→COL
COL→
COL+
Colct
Collect
Symb1 Symb2
Colσ
Comb
CON
Symb COMBsymb ,m COMBn, symb COMBsymb ,symb
Cond
Rarray constant
Carray constant Name
IDN
Conj
Access …µCONIC Input/Output None
Conic
Conlib
Const
Constants
Rarray Carray Symb CONJsymb
Cont
Convert
Corr
X1unitssource X2unitstarget X3unitstarget
Access Flags
COS
Cosh
COV
Crdir
Cross
Cswp
…µCR
Curl
Cyclotomic
Cylin
COL+, COL-, Rswp
→PX
Darcy
Date
#n, #m
→DATE
DATE+
Dbug
→date
Ddays
DEC
Decr
« program » or program name
Dedicace
DEF
Define
Result See also
Delalarm
DEG
Degree
Name=exp Namename Name =expname
Delay
RCLALARM, Stoalarm
Delkeys
CR, OLDPRT, PRLCD, PRST, PRSTC, PRVAR, PR1
Access …µDEPND Input/Output
Depnd
Depth
Xkey1, ... ,xkey n
Desolve
Deriv
Dervx
Access …µDETACH Input/Output
DET
Detach
Attach
Diagmap
DIAG→
→DIAG
Diff
Diffeq
Disp
Access …µDIFFEQ Input/Output
DIR
Dispxy
Distrib
Obj List
FREEZE, HALT, INPUT, Prompt
DIV
DIV2
DIV2MOD
CURL, Hess
Divis
Divmod
Divpc
DIV2
TAYLOR0, TAYLR, Series
DERIV, DERVX, DESOLVE, ∂
Until testclause END
END
Dolist
Error
Doerr
List List n « program » Results Command Name List n+1
Domain
Dosubs
SIGNTAB, Tabvar
List « program » Command Name
DOT
DOLIST, ENDSUB, NSUB, Stream
CNRM, CROSS, DET, Rnrm
Access …µDRAW
Access …µDRAX Input/Output None
Draw
DRAW3DMATRIX
Droite
Drop
DROP2
Lagrange
Dropn
Dtag
DUP
DUP2
Dupdup
Dupn
Egcd
Edit
Editb
EGV
Egvl
Else
END
Endsub
ENG
EPSX0
Sign mantissa E sign exponent
Eqnlib
EQW
EQ→
Erase
ERR0
Errm
Errn
Euler
Eval
Exlr
Obj See above
→NUM
Ex,y = excosy + iexsiny
EXP&LN
EXP
Expan
EXP2HYP
EXP2POW
Expand
Expandmod
COLCT, EXPAND, ISOL, QUAD, Show
Result X2+X
Expfit
Expln
Expm
Eyept
F0λ
Fact
Factor
…µF0λ
Modsto
Factormod
Factors
FAST3D
Access …µFANNING
Fanning
Access …µFAST3D Input/Output None
Access ! Test LL FC ? C
Fcoef
FC?
Fdistrib
FFT
CF, FC?, FS? FS?C, SF
Distrib
Findalarm
Access …µFINISH
Filer
Finish
Flasheval
Floor
FONT6
Xunit Nunit Symb FLOORsymb
FONT7
FONT8
FONT→
→FONT
For
Fourier
Free
Freeze
Xunit Yunit Symb FPsymb
Froots
FS?
Display Area Value Code
CLLCD, DISP, Halt
CF, FC?, FC?C, FS?C, SF
FS?C
Function
Exlr
Access …µFUNCTION
Fxnd
Gamma
Gauss
Gbasis
AXQ, QXA
GCD
Gcdmod
GET
GCDMOD, EGCD, IEGCD, LCM
GETI, PUT, Puti
Geti
Objget
GOR
Grad
GET, PUT, Puti
#n #m Grob1
Gramschmidt
Graph
Greduce
DEG, RAD
Access …µGRIDMAP Input/Output None
Access …µ→GROB
Gridmap
→GROB
Grob
Grobadd
Gxor
→LCD, LCD→
Hadamard
Halftan
Halt
GOR, REPL, SUB
Head
HEADER→
→HEADER
Help
Hermite
Hess
HEX
CURL, DIV
Hilbert
Histogram
Access …µHISTPLOT Input/Output None
Histplot
HMS
HMS+
HMS→
→HMS
… & 9L HMS +
… &9L HMS →
Iabcuv
Home
Horner
Ibasis
Ibernoulli
IBP
ABCUV, Iegcd
Ichinrem
IDN
Results See also
Chinrem
IDIV2
Iegcd
DIV2, Iquot
Ax+by=c
ELSE, END, IFERR, then
Iferr
Case
∑ Y k e
Ifft
IFT
Ifte
Ilap
Obj It depends
Image
LAP, Lapl
…ßIM
Rarray Carray Symb IMsymb
Incr
Indep
Inform
Global Global xstart xend
Resets
Title Format Resets Init → vals
Input
Init
PROMPT, STR→
Stack prompt Commandline prompt Result
INT
Integer
Intvx
INV
INTVX, Risch
Iquot
Command INVMOD2 Result
Invmod
Iremainder
Isol
Isom
Symb1 Global Symb2
ISPRIME?
Jordan
Mkisom
NEXTPRIME, Prevprime
KER
Kerrm
KEY
BASIS, Image
→KEYTIME
Access …µKEYEVAL Input/Output
Keyeval
KEYTIME→
Kget
Kill
Label
Access …µ→LANGUAGE Input/Output
Lagrange
LANGUAGE→
→LANGUAGE
LAP
Lapl
Last
Objn Obj1
Lastarg
LCD→
→LCD
LCM
Lcxm
Ldec
Lgcd
Libeval
Libs
HERMITE, Tchebycheff
Lim
Limit
LIN
Title, nlib, nport, ...,title, nlib, nport
Line
Σline
Linfit
Model Form of Expression
Linin
Linsolve
LIST→
→LIST
Σlist
List
Πlist
Access …¹ ¹is the rightshift of the Qkey
LNP1
Lname
Lncollect
Local
LOG
Ln x +
Symb LNP1symb
Access !Ø Input/Output
Logfit
Access …µLR Input/Output
Full Command and Function Reference 3139
LSQ
Intercept x Slope x
Lvar
MAD
DET, INV
Lname
Access …µMAP Input/Output
Main
Mant
MAP
Access …µ↓MATCH Input/Output
Access …µ↑MATCH
↑MATCH
Symb Symb symb
Maths
Matr
MAX
Symb Symb , symb
Maxr
Maxσ
Mcalc
Input/Output See also
Mean
MEM
Menu
BINS, MAXΣ, MINΣ, SDEV, TOT, VAR
Menuxy
RCLMENU, Tmenu
Merge
MIN
Minehunt
MENU, Tmenu
MINIFONT→
→MINIFONT
Minit
Minr
Minσ
Mitm
Mkisom
00000000000E-499
MOD
Modsto
Modular
Mod y
Molwt
Mroot
Msgbox
Name Or xunit String
Mslv
Msolvr
Multmod
Muser
→NDISP
EQNLIB, MCALC, MINIT, MITM, MROOT, MSLV, Muser
Access …ß NEG ßis the rightshift of the 1key
Ndist
Ndupn
NEG
Nextprime
Newob
Next
Access ! Test L not Is the leftshift of the Nkey
NIP
Not
Noval
Nsub
→NUM
NUM
…µNΣ
OBJ→
Numx
Numy
OCT
OFF
Oldprt
… &NL OBJ →
Openio
Access …µOLDPRT
Access …µOPENIO
Order
Over
Memory Directory Lorder is the leftshift of the Nkey
Global1 ... globaln
P2C
PA2B2
Parametric
Xmin, ymin, xmax, ymax, indep, res, axes, ptype, depend
Access …µPARITY Input/Output
Parity
Parsurface
Partfrac
Path
Pcar
Pcoef
Home directoryname 1 ... directoryname n
Pcov
Access …µPCONTOUR Input/Output None
Pcontour
Pdim
Perinfo
Perm
Xmax, ymax
Pertbl
Peval
Pgdir
Pick
Pict Command Puts the name Pict on the stack
Pict
Picture
Pinit
Access …µPKT
PIX?
Pixoff
Pixon
Plot
Plotadd
Pmax
Pmin
Pmini
Polar
Finds the minimal polynomial of a matrix
An nxn matrix a
Access …µPOLAR
Polynomial
POP
Form of Current Plotting Action Equation
Input An expression raised to a power
POS
Potential
Powexpand
Powmod
PR1
Predv
Predx
…µPR1
Object
Predy
Preval
Prevprime
Prlcd
Prompt
Promptsto
Proot
Propfrac
Global→
Prompt STO
Prst
Prstc
Prvar
Name Name1 name2 Nport global
Psdev
PSI
Psi
MEAN, PCOV, PVAR, SDEV, TOT, VAR
Ptayl
Ptprop
Purge
Symb String or x or xunit or name
Push
PUT
Global Global1 ... globaln
CLEAR, CLVAR, NEWOB, Pgdir
This command sequence
Puti
Obj put List
Obj put List Name
MEAN, PCOV, PSDEV, SDEV, VAR
Pvar
Pvars
Access …µPVARS Input/Output
Nport namebackup Memory
Pview
Pwrfit
+ c/d*i
PX→C
→Qπ
Quad
̟ + c/ d* ̟
+ c/ d
LQ, LSQ
Quot
Quote
Arithmetic, !ÞPOLYNOMIAL !«
Quotient of the Euclidean division
QXA
RAD
Rand
Rank
Access …µRATIO
Ranm
Ratio
LQ, LSQ, QR
Rceq
RCI
Rcij
Steq
Rclalarm
RCL
FINDALARM, Stoalarm
Rclf
Rclkeys
Rclmenu
Rclvx
Rclσ
Rcws
RDM
…ßL RE
RDZ
COMB, PERM, Rand
Recn
Rect
Recv
REsymb
Remainder
Rename
Quot
New name Old name
Reorder
Repeat
Repl
END, While
Plot Type Default Interval
RES
String target
String String result
Restore
Backup To restore a
Resultant
Slopefield
Risch
Revlist
Rewrite
RKF
Rkferr
Is the rightshift 3key
Problem for a differential equation
Rkfstep
RLB
RND
RLB, RR, RRB
RNDsymb
Rnrm
Roll
Rolld
Romupload
Root
ROT
ROW
3207
→ROW
ROW+
ROW→
Access … ÃL Byte RRB Is the rightshift of the 3key
RPL
RRB
RL, RLB, RRB
Rref
Rref
Rrefmod
RRK
− 2 t + t 2
Rrkstep
RKF, RKFERR, RKFSTEP, RRK, Rsberr
Rsberr
RSD
Access … Ã R → B
Rswp
Rules
DET, IDN
Same
→R, IM, RE
180/̟x
→Dsymb
Access …µSCALEH Input/Output
Sbrk
Scale
Scaleh
AUTO, SCALEH, Xrng
Scatrplot
Scatter
Access …µSCATTER Input/Output None
Schur
SCI
Sclσ
Sconj
Scroll
Sdev
Send
DOSUBS, Stream
SEQ
Series
Server
Seval
Show
Sidens
Sigma
X1/cm3
Access !ÖDERIV L
Access !ÖDERIV LL
Sigmavx
Sign
Signtab
SIMP2
SIGNsymb
ABS, MANT, Xpon
Simplify
SIN
Sincos
Sin z
Sinh
Sinv
Size
Sinh z
SLB
Slopefield
String Integer List Vector
Symb Grob
NEG, SCONJ, Sinv
Sneg
Snrm
Solve
Solveqn
Sort
Solver
Solvevx
Sphere
Srad
Square Analytic Function Returns the square of the argument
SQsymb
SRB
Srecv
COND, SNRM, Trace
ASR, SL, SLB, SR
Access …µSRECV
Srepl
SST
SST↓
Step
Start
STD
Access …µSTIME
Step
Steq
Stime
Stoalarm
Access Input/Output
STO
Buflen
Access …µSTOF
Stof
Stokeys
Óis the rightshift of the 9 key
STO+
Store
Stovx
STO/, +
Stoσ
STR→
→STR
DTAG, EQ→, LIST→, OBJ→, →STR
Stream
Strm
Sturm
List Obj Result
Sturmab
Stws
SUB
Sturmab
Subst
Subtmod
X1, y1 X2, y2
CHR, GOR, GXOR, NUM, POS, REPL, Size
SVD
SVL
Swap
DIAG→, MIN, SVL
SYST2MAT
Syseval
Sylvester
Tabval
100 y
X, symb
Symb 1, symb
Tabvar
→TAG
Tail
→ARRY
TAN
TAN2CS2
TAN2SC
Tan z
TAN2SC2
Tanh
Tanh z
TANHsymb
TAYLOR0
Taylr
Tchebycheff
HERMITE, Legendre
Tcollect
Tdelta
Tests
TDELTAsymb1, symb2
Teval
Texpand
Text
Then
Ticks
Time
→TIME
Tinc
Tlin
Tline
TINCsymb1, symb2
SIMPLIFY, TCOLLECT, Texpand
Tmenu
TOT
Trace
Tran
Transio
Trig
Effect
BAUD, CKSM, Parity
Trigcos
Trigo
Trigsin
Trigtan
CONJ, Tran
TRN
Trnc
Trunc
Truth
TRNCz
TRNCsymb
Tstr
Access …µTRUTH Input/Output None
Tsimp
Tvars
TVM
Tvmbeg
Tvmend
Builtin Commands
Type
Ubase
Object Type Number User objects
Ufact
UFL1→MINIF
Unassign
Unassume
Unpick
Unbind
→UNIT
Unrot
Until
Updir
Utpc
Utpf
Utpn
UTPF, UTPN, Utpt
UTPC, UTPN, Utpt
UVALsymb
Utpt
Uval
Flag -16 clear Rectangular mode Flag -16 set Polar mode € y
Coordinate System -16, Complex Mode
X1, x2
X1, x2, ..., xn Xn-2
Vandermonde
VAR
Access …µVERSION Input/Output
Vars
VER
Version
Variable name Contents opened in the command line Editor
Variable name Contents opened in the most suitable Editor
Visit
Visitb
Wait
Access ! L in Wait Is the leftshift of the Nkey
Vtype
Wireframe
While
While testclause Repeat loopclause END
While Repeat
Wslog
Access …µWSLOG
Access …µΣX Input/Output
ΣX2
Log4 ... log1
ΣX2
Xcol
Xget
Xmit
XOR
Access …µXMIT
Xnum
Xpon
Xput
XPONsymb
MANT, Sign
Command XQ.3658 Results 1829/5000
Command XQ.3658 Results √19/142
Xrecv
Xrng
Access …µXSEND
Xsend
Xserv
Input/Output RPN
Xvol
Xxrng
ΣXY
ΣX*Y
ΣY2
ΣY2
Ycol
…µΣY
AUTO, PDIM, PMAX, PMIN, Xrng
Yrng
Yslice
Yvol
Yyrng
Zeros
EYEPT, XVOL, XXRNG, YYRNG, Zvol
Power
Access Q
Zfactor
Zvol
Where
√ Square Root
Zsymb
Symbz
SQ, , Isol
Access …Á Áis the rightshift of the Ukey
Integrate
? Undefined
Lower limit, upper limit, integrand, name
∞ Infinity
Summation
Sigma Plus
Results -π/2
Access …µΣ+ Input/Output
Access …µΣ Input/Output
Sigma Minus
X 2, …, x m 1, …, x 1 m x n 1, … , x n m
∂ Derivative
Factorial
14159265359…
Symb Name Xunit
Percent
Unit attachment
Xy/100
Symb,x
Type Object Description
« » Program delimiters
Less than
≤ Less than or Equal
#n1 #n2
Greater than
≥ Greater than or Equal
≠ Not equal
Multiply
≠ symb
+ Add
Subtract
Divide
= Equal
Y1/unit
== Logical Equality
Store
→ Create Local
DEFINE, RCL, →, STO
… É
Semicolon
CLEAR, DROP, DROPN, DROP2
CAS Settings
Selecting CAS Settings
Points to note when choosing settings
CAS directory, Casdir
Computer Algebra System
Using the CAS
Examples and Help
Compatibility with Other Calculators
Extending the CAS
Computer algebra command categories listed by menu
Arithmetic commands
Algebra commands, …×
Arithmetic Integer commands, !ÞINTEGER
Other Arithmetic commands, !Þ
Arithmetic Modulo commands, !Þ Modulo
Arithmetic Permutation commands, !Þ Permutation
Calculus commands
Exp and Lin commands, !Ð
Matrixrelated commands
Create, !Ø Create
Operations, !Ø Operations
Quadratic form, !Ø Quadratic Form
Linear Systems, !Ø Linear Systems
Linear Applications, !Ø Linear Appl
Eigenvectors, !Ø Eigenvectors
Symbolic solve commands, !Î
Trigonometry commands
Other Trigonometry commands, …Ñ
Hyperbolic, …Ñ Hyperbolic
Convert commands, !Ú
Unit conversion tools, !Ú Units Tools
Base conversion tools, !Ú Base
Trigonometric conversions, !Ú Trig Conv
Other CAS operations, …µ
Matrix convert, !Ú Matrix Convert
CAS utility operations
CAS menu commands, …µ
These commands display menus or lists of CAS operations
Equation Reference
Equation Reference
FLUIDS, 24 *********************3
Columns and Beams
Variable Description
Σcr Critical stress Σmax Maximum stress
Internal bending moment at
Elastic Buckling 1
Eccentric Columns 1
Equations
Example
Equation
Simple Deflection 1
Simple Slope 1
Simple Shear 1
Solution V=624.387lbf
Simple Moment 1
Cantilever Moment 1
Cantilever Deflection 1
Cantilever Slope 1
Solution V=200lbf Equation Reference
Cantilever Shear 1
Relative permeability
Electricity
Relative permittivity
Ohm’s Law and Power 2
Coulomb’s Law 2
Wire Resistance 2
Solution V1=80V
Solution I1=5.6250A
Voltage Divider 2
Series and Parallel C 2
Solution R=0.175
Series and Parallel R 2
Solution V=50V
Series and Parallel L 2
Capacitive Energy 2
Inductive Energy 2
RLC Current Delay 2
Solution q=0.0020C Equation Reference
DC Capacitor Current 2
Capacitor Charge 2
Solution V=3.2968V
DC Inductor Voltage 2
RC Transient 2
RL Transient 2
Solution I=0.0072A
Resonant Frequency 2
Plate Capacitor 2
Cylindrical Capacitor 2,19
Solenoid Inductance 2
Toroid Inductance 2
Sinusoidal Current 2
Fluids
Sinusoidal Voltage 2
Pressure at Depth 3
Bernoulli Equation 3
Reynolds number
Initial and final velocities
Flow with Losses 3
Forces and Energy
Flow in Full Pipes 3
Linear Mechanics 4
Hooke’s Law 4
Angular Mechanics 4
Centripetal Force 4
1D Elastic Collisions 4
Drag Force 4
Law of Gravitation 4
MassEnergy Relation 4
Gases
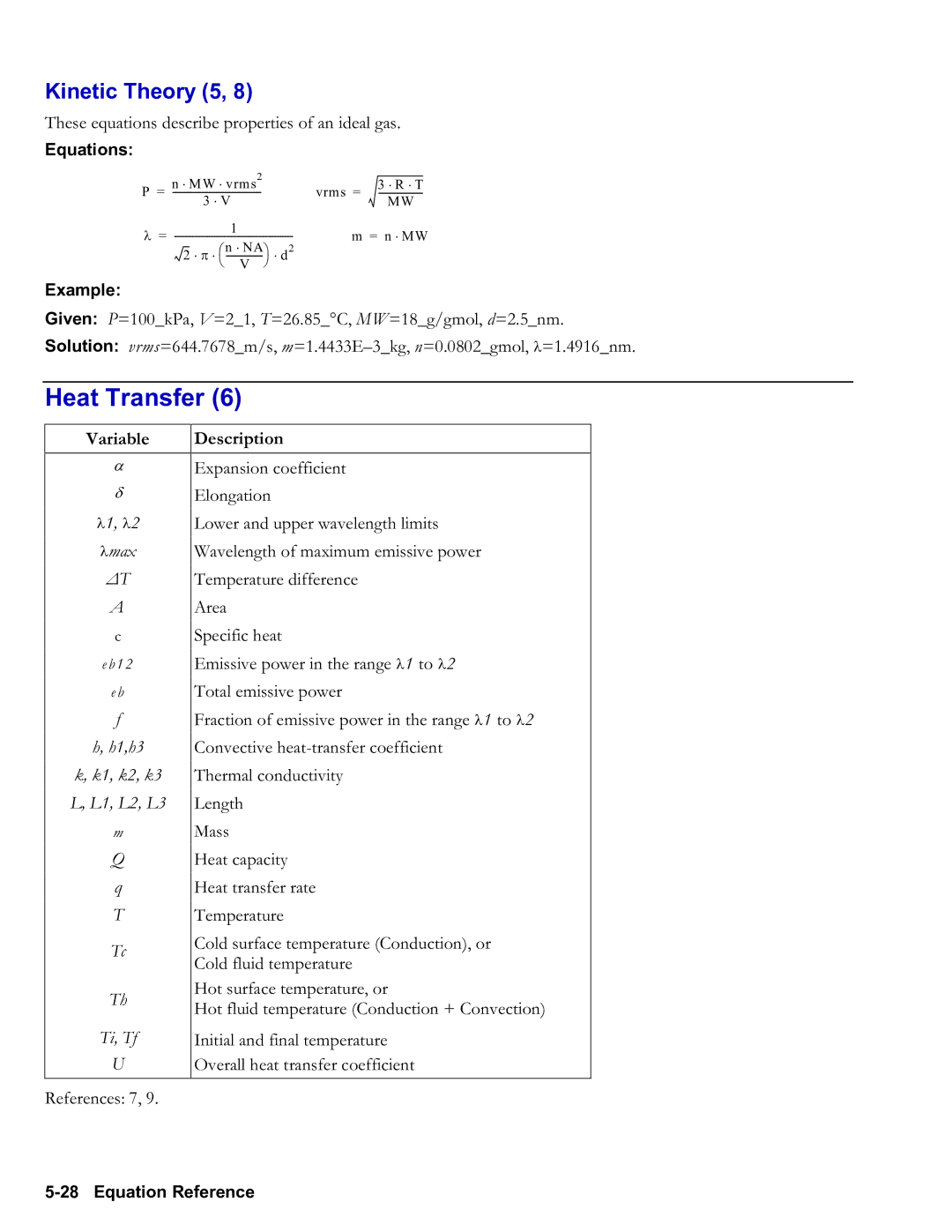
Ideal Gas Law 5
Solution Vf=21
Ideal Gas State Change 5
Isothermal Expansion 5
Polytropic Processes 5
Real Gas Law 5
Real Gas State Change 5
Heat Transfer
Kinetic Theory 5
Conduction 6
Heat Capacity 6
Thermal Expansion 6
Convection 6
Conduction + Convection 6
Magnetism
Black Body Radiation 6
Fba
Ia, Ib
Straight Wire 7
Force between Wires 7
Magnetic B Field in Toroid 7
Solution B=0.0785T
Magnetic B Field in Solenoid 7
Motion
Projectile Motion 8
Linear Motion 8
Object in Free Fall 8
Angular Motion 8
Circular Motion 8
Terminal Velocity 8
Escape Velocity 8
Optics
Law of Refraction 9
Critical Angle 9
Brewster’s Law 9
Solution n2=1.5000
Spherical Reflection 9
Spherical Refraction 9
Thin Lens 9
Oscillations
Example Given L=15cm
MassSpring System 10
Simple Pendulum 10
Simple Harmonic 10
Conical Pendulum 10
Torsional Pendulum 10
Plane Geometry
Equations Example
Circle 11
Ellipse 11
Rectangle 11
Regular Polygon 11
Circular Ring 11
Triangle 11
Solid Geometry
Cone 12
Cylinder 12
Parallelepiped 12
Sphere 12
Solid State Devices
Saturation current density
Drawn mask length PN Step Junctions, or
Drawn gate length Nmos Transistors, or
Channel length JFETs
PN Step Junctions 13
Nmos Transistors 13
VDS
Bipolar Transistors 13
JFETs 13
Xdmax = ⋅ Vbi VGS + VDS
Minimum principal normal stress
Stress Analysis
Maximum principal normal stress
Stress on an Element 14
Normal Stress 14
Shear Stress 14
Mohr’s Circle 14
Example
Longitudinal Waves 15
Waves
Transverse Waves 15,1
Sound Waves 15
References
Page
Development Library
Development Library Command Reference
Obj String
Obj1 … objn Symb Obj1, ...,objn
Development Library
BetaTesting
String Obj Debug string Error list
String Obj
Code String
Library n
String Error list Edited string
Library Object String
LC~C
Ey,z.Et
→LST
Obj … obj Obj 1, ...,obj n
Pokearm
Obj 1 … obj n Obj 1, ...,obj n
→S2
String1 String2
Returns
String Global
Crlib Create Library Command
Xlib
Extension program
Masd The Machine Language and System RPL Compiler
Introduction
Starting Masd
Modes
Error messages Message Description
Errors
Format of the error list
Links
Labels
Extable
Constants
Expressions
Operator Priority
Macros and includes
Directive Description
Filename conventions
Compilation directive
!DBGINF directive
Docstr
Bit registers
Saturn ASM mode
CPU architecture
Skips
Other notes
New instructions
Skips instructions Equivalents
These instructions Are equivalent to
Tests
Saturn instructions syntax
Syntax Example
Syntax Example
DReg=hh
See above. This is only valid in emulated Saturn
Places the value of Exp in the code, on x nibbles
CARRY0
003AA Autousertest
ARM mode
ARM architecture
Instruction set
Description Form
Operation Assembler Action Flags
Armsat instruction
Offset Element
REG a
REG B
REG C REG D REG R0 REG R1 REG R2 REG R3 REG R4
System RPL mode
Instructions
Reals and system binary
Unnamed local variables
Token Description
Defines
Tokens
Code
LC 001 Gosbvl Outcinrtn ?CBIT=1.6
Turnmenuoff
LC 80100 Armsat
Disassemblers
Level
Entry Point Library Extable
Library
Nop
String String 1, ...,string n
Error and Status Messages A1
Messages Listed Alphabetically
Message Meaning # hex
C0C
A2 Error and Status Messages
Error and Status Messages A3
A4 Error and Status Messages
Σdat
Error and Status Messages A5
A6 Error and Status Messages
Error and Status Messages A7
C0E
C0B
C0D
A8 Error and Status Messages
Error and Status Messages A9
# hex Message General Messages
A10 Error and Status Messages
# hex Message
Error and Status Messages A11
OutofMemory Prompts
Stack Errors and Messages
A12 Error and Status Messages
Object Editing Messages
Equation Writer Application Messages
# hex Message FloatingPoint Errors
Error and Status Messages A13
Array Messages
Statistics Messages
Mode and Plot Input Form Prompts
A14 Error and Status Messages
Error and Status Messages A15
# hex Message 70F
A16 Error and Status Messages
73F
Error and Status Messages A17
75F
A18 Error and Status Messages
78F
Error and Status Messages A19
A20 Error and Status Messages
Advanced Statistics Messages
Error and Status Messages A21
80F
A22 Error and Status Messages
82F
Error and Status Messages A23
85F
A24 Error and Status Messages
87F
HP Solve Application Messages
Error and Status Messages A25
Statistics Help Messages
# hex Message Unit Management
Printing
Time Messages
Programmer’s Doerr
Error and Status Messages A27
A28 Error and Status Messages
System Flags Choose Box Prompts
Error and Status Messages A29
A30 Error and Status Messages
Error and Status Messages A31
A32 Error and Status Messages
Prompts
Error and Status Messages A33
A34 Error and Status Messages
Error and Status Messages A35
Statistics Prompts
Time and Alarm Prompts
A36 Error and Status Messages
Error and Status Messages A37
Symbolic Application Prompts
A38 Error and Status Messages
Error and Status Messages A39
Plot Application Prompts
A40 Error and Status Messages
Error and Status Messages A41
A42 Error and Status Messages
Solve Application Prompts
Error and Status Messages A43
A44 Error and Status Messages
Error and Status Messages A45
CAS Messages
A46 Error and Status Messages
Error and Status Messages A47
A48 Error and Status Messages
Filer Application Messages
DF0A DF0B DF0C DF0D DF0E DF0F
DF1A DF1B DF1C DF1D DF1E DF1F
Error and Status Messages A49
Constants Library Messages
A50 Error and Status Messages
Equation Library Messages
Minehunt Game Prompts
MultipleEquation Solver Messages
Error and Status Messages A51
Periodic Table Messages
Financial Solver Messages
Development Library and Miscellaneous Messages
A52 Error and Status Messages
Units
Tables of Units and Constants B1
B2 Tables of Units and Constants
Tables of Units and Constants B3
B4 Tables of Units and Constants
Tables of Units and Constants B5
Constant Full Name Value in SI Units
Properties of Elements
B6 Tables of Units and Constants
System Flags
Flag Description
Not used
System Flags C1
C2 System Flags
System Flags C3
C4 System Flags
System Flags C5
C6 System Flags
System Flags C7
Internal use only /0 occurred
C8 System Flags
User Flags
System Flags C9
Page
Reserved Variables D1
Contents of the System Reserved Variables
ΒENTER
Reserved Variables D3
Exited
D4 Reserved Variables
Reserved Variables D5
MHpar
Mpar
N1, n2
Nmines
BAR, etc
Reserved Variables D7
Prtpar
D8 Reserved Variables
S1, s2
Reserved Variables D9
Parameter Description Default Value Command
To use for input into the table
NUMX, Numy
D10 Reserved Variables
Reserved Variables D11
Var1 var2 … varm
Statistical Matrix for Variables 1 to m
EXPFIT, PWRFIT, or Logfit
D12 Reserved Variables
Casdir Reserved Variables
Modulo
D14 Reserved Variables
Technical Reference E1
Object Size
Object Type Size bytes
E2 Technical Reference
Symbolic Integration
Pattern Antiderivative
Technical Reference E3
→DEF Expansions
E4 Technical Reference
Order Operation
Technical Reference E5
International Standard publication No. ISO 31/l197 8 E
E6 Technical Reference
Group 1 Commands that cannot parallel process
Group 2 Commands that must use Dolist to parallel process
General rules for parallel processing
Parallel Processing with Lists F1
Group 5 Commands that set modes / states
Group 6 Oneargument, oneresult commands
Group 4 ADD and +
F2 Parallel Processing with Lists
Group 7 Two argument, one result commands
Group 8 Multipleargument, oneresult commands
Group 9 Multipleresult commands
Group 10 Quirky commands
Using Dolist for Parallel Processing
F4 Parallel Processing with Lists
$ & C
$ & D
$ & F
$ & +
Keycode Keystroke Definition
¥ ! &I
@ & ³ @ & O
~@ & O
Keyboard Shortcuts G3
~@ & Í @ &†
@%VARNAME% = varname
G4 Keyboard Shortcuts
MenuNumber Table H1
Menu Numbers
Syntax Example
Menus 0 through
H2 The MenuNumber Table
MenuNumber Table H3
Menus 118 through
H4 The MenuNumber Table
MenuNumber Table H5
Menus 178 through
BuiltIn Library Menus
Page
+ key
Command MenuPath Table
Key ALPHARS2 MTH NXT Prob
Command Type Library Size Keys Menu First
I2 The Command MenuPath Table
MTH List
Arith Modul
«MODULAR» Addtoreal
CAT Algb
I4 The Command MenuPath Table
LS&MODE Flag
PRG Test NXT NXT PRG NXT Modes Flag Chinrem
PRG NXT
CHR
I6 The Command MenuPath Table
CAT Delalarm
PRG NXT NXT Time Alrm Delay
RS&TIME NXT
PRG NXT NXT Time NXT
I8 The Command MenuPath Table
PRG NXT NXT Error Iferr
«POLYNOMIAL» EGV
MTH Matrx NXT
Else
I10 The Command MenuPath Table
I11
I12 The Command MenuPath Table
Calc Deriv NXT
«INTEGER»
PRG Brch NXT
«DIFF» NXT NXT
I14 The Command MenuPath Table
I15
I16 The Command MenuPath Table
I17
I18 The Command MenuPath Table
I19
I20 The Command MenuPath Table
I21
I22 The Command MenuPath Table
I23
I24 The Command MenuPath Table
I25
I26 The Command MenuPath Table
I27
I28 The Command MenuPath Table
Matrices Creat NXT NXT MTH Matrx Make NXT NXT
DUP Menuxy Version
CAT Vtype
XLIB~
I30 The Command MenuPath Table
SLV NXT
Alphalse
MTH NXT Const «CONSTANTS»
Σline
Σlist
PRG Test «TESTS» NXT
→ALG
I32 The Command MenuPath Table
Ascii Character Codes and Translations J1
Code Description
Character Codes
Code Trans. Description
J2 Ascii Character Codes and Translations
Index
Alpha keyboard
Calculator
59, 531
375 Preserving calculator status
Flags
132
Last argument
Memory
Nested structures
545
Programs
Range 3115
Serial communications
237, D3
Tagged objects
UPs